Percutaneous Radiologic Approach to Management of the Native Dialysis Fistula
More than 70% of American hemodialyzed patients with arteriovenous access undergo treatment using a prosthetic graft and only a few using a native fistula. The proportions are more or less the opposite in Europe and in Asia, where more than 70% of patients have a native fistula and some centers report a rate close to 100%. The American situation usually is explained by a higher proportion of diabetics (> 33% compared with < 20% in Europe) and blacks (40% versus 1%); however, the surgical literature also shows opposing schools of thought because statistics from 1994 show that fewer than 15% of native fistulae were created even in young, white, nondiabetic American patients.1 The recently published DOQI guidelines of the National Kidney Foundation strongly encourage American surgeons to “take a step back“ and to create more native fistulae.2
American radiologists treat many stenoses and thromboses of grafts but relatively few native fistulae. In contrast, many European radiologists deal with failing or failed native fistulae as often as with grafts because the less numerous grafts have clearly higher complication rates.3
 Pathophysiolgy
Pathophysiolgy
Stenosis, the most frequent complication of hemodialysis access, leads to thrombosis. Many mechanisms have been put forward to explain the genesis of these stenoses, but only direct venous trauma and turbulent flow have been proven to date. Any venous catheterization can induce subsequent stenosis, especially central veins, where most stenoses are located as a result of central catheters placed in intensive care units or for temporary dialysis. Subclavian catheters are particularly damaging and must be prohibited; the internal jugular approach induces fewer stenoses. Similarly, repeated peripheral venipunctures destroy the patient’s venous capital and preclude the creation of native fistulae when permanent hemodialysis becomes necessary. An overall culture of venous preservation should be instilled from the beginning in medical and nursing schools: Only veins of the dorsum of the hand or at the elbow should be punctured and cannulated—never the forearm veins. Turbulence generated at surgical anastomoses explains the development of stenoses at the venous anastomosis of grafts and also explains the frequent juxtaanastomotic stenoses in native fistulae. We still have much to discover, however, about the pathophysiology and prevention of stenoses in native fistulae.
 Diagnostic Fistulography
Diagnostic Fistulography
Indications
Any clinical abnormality indicates angiography with a view to its correction. As for grafts, stenosis-detection programs with the aim of prevention of native fistula thrombosis should be initiated in all dialysis centers. Stenoses can be detected by indirect signs, such as reduction of flow rate, recirculation, pressure measurements, or Doppler imaging.4–7 Clinical examination is the key detection method (see also Chapter 18). Clinical examination of native fistulae is easier to perform than in grafts. Most stenoses lie just under the skin and can be palpated as a tough cord with a firm, dilated vein upstream and a flat vein downstream. Pressure upstream from the stenosis, however, can be reduced by alternative flow into collaterals, which are also a sign of dysfunction for which the patient must be examined. Stenoses of the arterial inflow are responsible for an overall flat fistula, which is difficult to cannulate without the help of a tourniquet. Stenoses of the venous outflow are typically responsible for a “too convenient fistula,” which is much too visible and easy to puncture and involves subsequent increased compression times after dialysis and formation of venous aneurysms. These aneurysms, which are common in cannulation sites after some months of dialysis, are ideal examination points because the skin is even thinner at their level. An aneurysm under tension indicates an outflow stenosis, whereas an abnormally flat and depressible aneurysm indicates an inflow problem. In cases of a stenosis developing between the two cannulation sites, the “arterial” aneurysm is tough, whereas the “venous” aneurysm is soft. In addition, stenoses in cannulation areas can make routine needling impossible.
Technique
Diagnostic fistulography is nowadays performed as an integral part of a concomitant interventional procedure. The use of carbon dioxide as a contrast agent has become a viable alternative in cases of absolute contraindication to iodine injection, although imaging quality is poorer. The fistulography technique must be appropriate to the clinical situation. In cases of poor inflow (the most frequent situation in native fistulae), retrograde puncture of the brachial artery at the elbow is necessary to visualize the entire feeding artery and the arteriovenous anastomosis. This approach also must be used in the exploration of delayed maturation. In cases of outflow obstruction, antegrade puncture of the fistula is performed just beyond the anastomosis. In cases of high outflow or hand ischemia, complete visualization of the arteries of the limb is required to appreciate the possibility of reducing fistula flow or improving peripheral flow. This procedure may be performed by placing a blood pressure cuff on the upper arm; with the cuff inflated to suprasystolic level, contrast is injected into the fistula, which will reflux contrast up the inflow vessels. In difficult cases, puncture of the brachial artery also may be used, although the femoral route may be necessary for upper-arm fistulae. In cases of distal ischemia, acquisitions centered on the forearm and the hand must be performed before and during compression of the fistula to appreciate the respective roles of steal and arterial lesions in the process.
Results
Stenoses in forearm native fistulae predominate in the anastomotic area but can occur anywhere from the feeding artery to the superior vena cava.8 Stenosis in the arterial system is graded according to the narrowing of the diameter, the reference being the diameter of the immediately upstream or downstream normal vessel. Stenosis over 50 or 70% is considered significant, depending on the location of the stenosis and the reporting investigator. In hemodialysis access, the reference diameter can be difficult to determine in irregular and aneurysmal veins or at the confluence of two vessels. Measurement of the diameter of a stenosis can be impossible in cases of heterogeneity, stricture, or superimposition. In such cases, a pullback pressure measurement is more reliable and not operator dependent. We suggest grading according to the percentage of systolic pressure drop through the stenosis. It is, however, still not the perfect tool. This pressure gradient is fully reliable when no collaterals are visible upstream from the stenosis, but it underestimates the obstruction if there are collaterals leaving the fistula before the stenosis that reduce the upstream pressure. On the other hand, upstream pressure is abnormally high and leads to overestimation of the stenosis in high flow fistulae (>1.5 L/minute). This is less of a problem because high flow is a contraindication for dilation. When there is no obvious stenosis on the angiogram, despite clinical abnormalities, pullback pressure measurement from the superior vena cava to the anastomosis is indicated. An abrupt systolic pressure loss of more than 50% would indicate either a too high flow fistula or a stenosis that was overlooked. Similarly, visualization of collaterals means either stenosis or a high-flow fistula. A 50% pressure drop is normal at the arteriovenous anastomosis.
 Stenosis Treatment in Patent Fistulae
Stenosis Treatment in Patent Fistulae
Dilation
Stenosis must be treated when it results in insufficient dialysis or clinical impairment or if it threatens access patency. Insufficient dialysis is diagnosed by biological examinations. Clinical impairment includes insufficient inflow with vacuum phenomenon during dialysis, difficulties in cannulation, venous hyperpressure, arm edema, increased bleeding after needle removal, distal ischemia, aneurysms, pain, and skin necrosis. Insufficient inflow with vacuum phenomenon impairing dialysis is a late sign, and the fistula should be referred immediately for angiography and stenosis correction when it occurs. Although the relation of access thrombosis to stenosis is proven, because an underlying stenosis is unmasked in all cases of native fistula thrombosis, it must not be overestimated. Many studies are in progress to determine when a stenosis must be corrected solely for the prevention of thrombosis, but most of these studies are focused on grafts. Such stenosis detection and grading techniques are based mainly on direct or indirect measurement of flow rates and pressures in the access. It is recommended that prophylactic dilation of all hemodynamically confirmed stenoses greater than 50% at the venous anastomosis of grafts be performed in the setting of a clinical indicator of failure (DOQI guidelines).2 Only very tight stenoses threaten native fistula patency, however, and most of these are easily detectable by simple physical examination. In our opinion, a greater threshold of 70% should be enforced to decide when to dilate these asymptomatic stenoses in native fistulae simply for prevention of thrombosis.
Similarly, apparent stenoses of the anastomosis of native fistulae must be dilated only in cases of poor inflow because dilation of a well-functioning fistula could lead to secondary high flow or steal phenomenon. Alternatively, it could accelerate the stenosing process and lead to early restenosis that could be more severe and lead to thrombosis. In our experience, we have seen native fistulae with flow rates over 1.5 L per minute despite a 70% stenosis of the anastomosis. Of course, it would have been a major error to dilate them.
Dilation is an outpatient procedure. Hemodialysis fistulae are made to be punctured and must be used for the introduction of dilation catheters. Puncture of the fistula for dilation is performed with the patient under local anesthesia in the direction of, but far enough from (5 cm), the presumed stenosis. An antegrade approach should be used for stenoses located far enough from the anastomosis, and a retrograde approach should be used for stenoses close to the anastomosis. A 16 to 18G needle and a 0.035-inch guidewire offer good support for placement of a 6 to 9F introducer sheath. A small subcutaneous tunnel between the entry points of the skin and vein will facilitate hemostasis after the procedure and may decrease the risk of pseudoaneurysm formation. We prefer soft-tipped guidewire (e.g., a Bentson) to traverse stenoses. Angled catheters are helpful for selective catheterization of branches. Hydrophilic guidewires are effective but must be used carefully because of their propensity to enter vessel walls in stenosed areas and to create dissections. Heparin may be injected (2000-3000 U) once a guidewire has passed through the stenosis, but it is in fact necessary only in small-diameter or low-flow fistulae. The diameter of the dilation balloon should be equal to or 1 mm greater than the diameter of the immediately upstream or downstream normal vessel (take the smaller one first in cases of discrepancy). The dilation balloon then is placed through the stenosis under fluoroscopic guidance. It is inflated with a manometer filled with contrast medium diluted to 75%. In cases of major allergy to iodine, the balloon can be inflated with gadolinium (balloons can burst). Pressure is slowly increased to abolish the waist of the stenosis on the balloon, the edges of which must be completely parallel. The inflated balloon is left in place for 1 to 3 minutes. Dilation is often painful locally. Local anesthesia can be performed when stenoses are just under the skin. Otherwise, neuroleptanalgesia may be necessary.
Venous stenoses are often hard, and high-pressure balloons with burst pressures over 25 atmospheres may be necessary (e.g., Blue Max, Meditech, Natick, MA, or Centurion, Bard, Covington, GA). Balloon bursting during dilatation must be avoided because of the risk of fragmentation and vessel rupture; however, such complications are rare, and balloon bursting is preferable to residual stenosis. Immediate postdilation angiography, with the guidewire left in place through the dilated area, may show several possibilities:
1. No residual stenosis and no parietal damage: the procedure is complete.
2. Minor parietal damage: 3 to 5 minutes’ low-pressure ballooning is performed to try to smooth the vessel wall.
3. Rupture with clear extravasation of contrast medium and creation of a hematoma: The dilation balloon must be rapidly reinflated to 2 atmospheres for repeated periods of 10 minutes.
4. Residual stenosis: If no parietal damage occurs, a new dilation is performed with a balloon 1 mm greater in diameter. At this stage, a residual stenosis of less than 30% is acceptable only if it results from the first dilation ever performed in this stenosis. If greater than 30% residual stenosis is present, a new dilation should be performed using a larger balloon, but more than 2 mm overdilation is not recommended. Stenosis recoil is possible, especially in central veins. Recoil means that the vessel wall collapses and that the stenosis reforms as soon as the balloon is removed, despite the absence of waisting on the inflated dilation balloon. Recoil is treated by stent placement.
In cases of early recurrence of a previously dilated stenosis (< 4 months), it is necessary to be more demanding with regard to the result, and absolutely no residual stenosis should be accepted: Greater overdilation or stent placement must be considered to achieve the perfect result. Once the dilation has been performed, the catheters and introducer sheath are removed. The puncture site must be compressed as gently as possible to stop bleeding without stopping flow through the fistula. The outpatient then can get up, and the vascular access is immediately usable for hemodialysis.
Problem Solving
Stenoses resistant to 25 atmospheres pressures are infrequent. In our experience, they occur in 1% of forearm fistulae and in 5% of upper-arm fistulae (final arch of the cephalic vein) (Fig. 21–1).9 Atherectomy catheters have been reported to be of some value in such cases. Cutting balloons10 are a more recent and valuable alternative for tight stenoses, but they are not available in diameters greater than 4 mm. A newly available device, the “Redha-cut” (Sherinemed, Utzenstorf, Switzerland), also might be of some help. Stenoses of the feeding artery and of the arteriovenous anastomosis may be impossible to traverse using a regular retrograde fistula approach. Antegrade puncture of the brachial artery and catheterization of the feeding artery are feasible (Fig. 21–2). In the setting of distal ischemia, dilation has no place if the arteries are normal and if distal ischemia is due simply to the arterial steal of the fistula. In contrast, dilation of an arterial stenosis before or after the arteriovenous anastomosis can improve distal flow significantly and cure the patient.
Complications of Dilation
Dilation-induced rupture can occur at a low pressure and is not systematically linked to the balloon bursting. The risk of rupture can be suspected when the waisting on the balloon suddenly disappears, whatever the level of pressure. The patient often reports a sudden acute pain at the dilated site as soon as the balloon is deflated. If rupture is confirmed, the balloon must be reinflated quickly to 2 atmospheres and left in place for 10 minutes. If it was previously injected, heparin must be neutralized by an injection of protamine (except in patients taking insulin), and the hematoma is compressed manually if it is clinically accessible. If extravasation continues despite three periods of 10-minute balloon tamponade, a stent must be placed. In our experience, rupture during dilation occurred in 8% of Brescia-Cimino fistulae and in 15% of upper-arm fistulae.9 Most were controlled by prolonged, balloon inflation, and few required a stent. The final arch of the cephalic vein (Fig. 21–3), the axillary outflow of transposed brachiobasilic veins (Fig. 21–4), and the postanastomotic area of forearm fistulae at the wrist are the most likely to rupture.
Pseudoaneurysms are often the consequence of a dilation-induced rupture that was initially controlled but reopened and was limited in its expansion (see Fig. 21–4). Such aneurysms can be treated by placement of a stent through the base. Aneurysms in cannulation areas can be the consequence of reopening the entry point of the introducer sheath as well as reopening of a dialysis needle. Risk increases with introducer size, with pressure in the vascular access, and in a patient who is receiving anticoagulants. Surgical treatment is generally preferable to stent placement in this location.
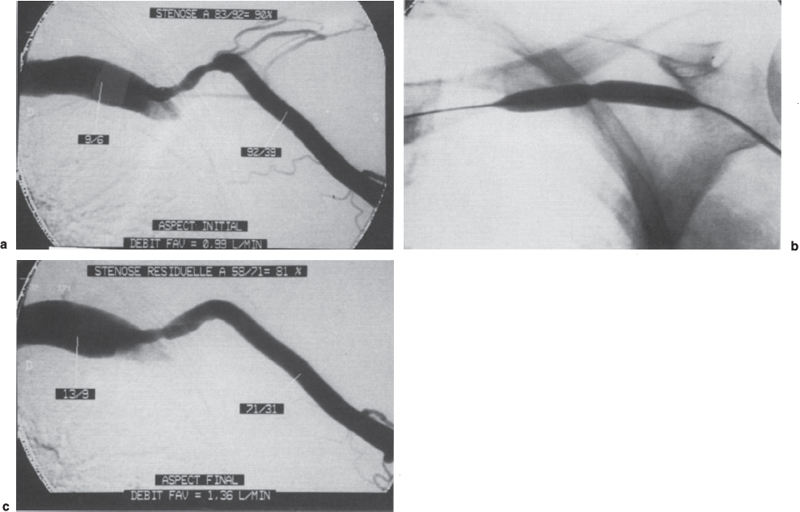
FIGURE 21–1. (a) Angiography of this brachiocephalic fistula was performed for increased venous pressure. Stenosis of the final arch resulted in a 90% decrease in systolic pressure [(92–9):92 = 90%] between the cephalic vein and the subclavian vein. The fistula flow rate was 0.99 L/min measured by thermodilution with a Swan-Ganz catheter. (b) It was impossible to abolish residual waisting on the 7-mm high-pressure balloon, despite prolonged inflation to 25 atm. (c) The significant angiographic residual stenosis with a residual 81% decrease in systolic pressure was therefore not a surprise. The increase in the flow rate to 1.36 L/min was more surprising, and the conclusion was that successful dilation of the stenosis would have led to restoring a fistula with too high a flow. The possibilities of surgical inflow reduction had to be considered before using an atherectomy catheter or a cutting balloon on the resistant stenosis.
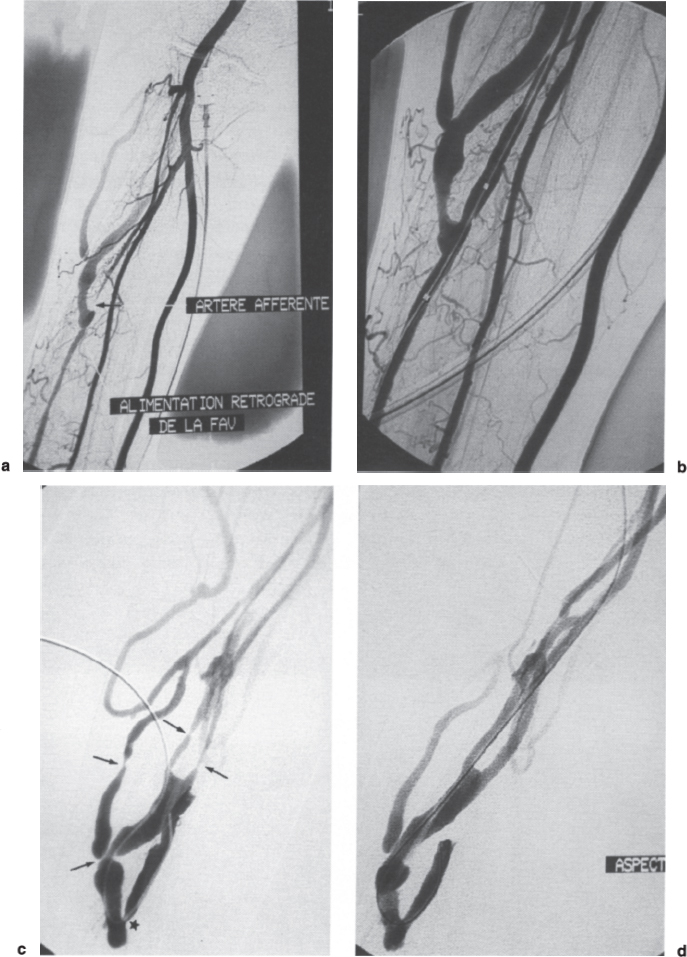
FIGURE 21–2. (a) Angiography of this radiocephalic fistula was performed for insufficient inflow and was therefore achieved by direct puncture of the brachial artery at the elbow. The fistula was fed by retrograde flow from the distal (and irregular) radial artery because the proximal radial artery was occluded just above the anastomosis (arrow). Despite the overall poor quality of the radial artery, it was decided to try to dilate it because it was the only way to save the fistula. (b) Antegrade puncture of the brachial artery at the elbow and selective catheterization of the radial artery were the only possible approach. Recanalization was fairly easy and dilation to 5 mm was performed, yielding this relatively satisfactory result. (c) New angiography was performed 13 months later because of venous hypertension. There were four stenoses (arrows) on the vein but only a mild restenosis on the artery close to the anastomosis (star). (d) The final result after dilation was satisfactory on this fistula, which has now been subsequently used for 6 months.
Thrombosis can occur during dilation if the introducer is occlusive or if the balloon is left inflated for too long in a nonanticoagulated patient. Dilation of a juxtaanastomotic stenosis in forearm fistulae can cause transient occlusion of the feeding artery by a mechanism of extrinsic compression (Fig. 21–5). When dilating such stenoses close to the anastomosis, a guidewire must be left through the anastomosis to allow rapid reopening of the artery if necessary. Secondary thrombosis some hours or days after dilatation is vexing and can be explained by excessive final compression, compressive hematoma, overlooked or insufficiently dilated stenoses, secondary clot formation at the dilated site, or stenosis induced at the entry point of the introducer sheath.
Bleeding during dilation is no longer a problem when using introducer sheaths. As after a dialysis session, the cannulation site can subsequently reopen, and control of such bleeding by manual compression is a part of the basic education of hemodialyzed patients. When a large introducer sheath has been used (9 F), U-shaped suturing can effectively prevent such secondary bleeding and pseudoaneurysms.11 The other preventive method is to use the femoral route.
Infection is a potentially fatal complication that has been reported in several series because hemodialyzed patients have immunological disorders. Systematic treatment with antistaphylococcic antibiotics is required when a difficult dilation procedure has been prolonged, when several cannulations were necessary, when clots are present, when the fistula was recently operated on, and above all in cases of a breach in sterile technique.
Contraindications to Dilation
Local infection and severe hypocoagulability states are absolute contraindications to percutaneous treatment. Dilation, however, can be planned after correction of these disorders. Concomitant arterial steal syndrome is an absolute contraindication to treatment of any stenosis located in the fistula downstream from the arterial anastomosis because treatment would increase both flow in the fistula and subsequent steal.
Immature (< 2 months) fistulae are indications for surgery if the stenosis is located in the anastomotic area. When the underlying stenosis is located several centimeters from the stenosis on the venous outflow or on the feeding artery, however, surgical creation of a new anastomosis downstream from the venous stenosis or upstream from the arterial stenosis would not leave enough vein for cannulation. In such cases, gentle dilatation is the only possibility to save the fistula, although cannulation and catheterization of an immature fistula also can generate stenosis. Isolated stenosis within 10 cm of the wrist in a Brescia-Cimino fistula can be dilated, but there is a risk of early restenosis. In contrast, surgical creation of a new anastomosis downstream from the stenosis is simple, minimally invasive, and more durable. Dilation remains the best treatment, however, when the radial artery above the anastomosis is occluded (Fig. 21–6) in which the fistula is fed by the ulnar artery through the palmar arch and retrograde flow from the distal radial artery. Long (> 5 cm) stenoses and chronic occlusions can be successfully dilated (Fig. 21–7), but stents are frequently needed in cases of recanalization at the elbow and in the final arch of the cephalic vein, allowing discussion of surgical revision. Some forearm fistulae whose elbow drainage relies only on collaterals can work relatively well and allow correct dialysis for years despite chronic hyperpressure.
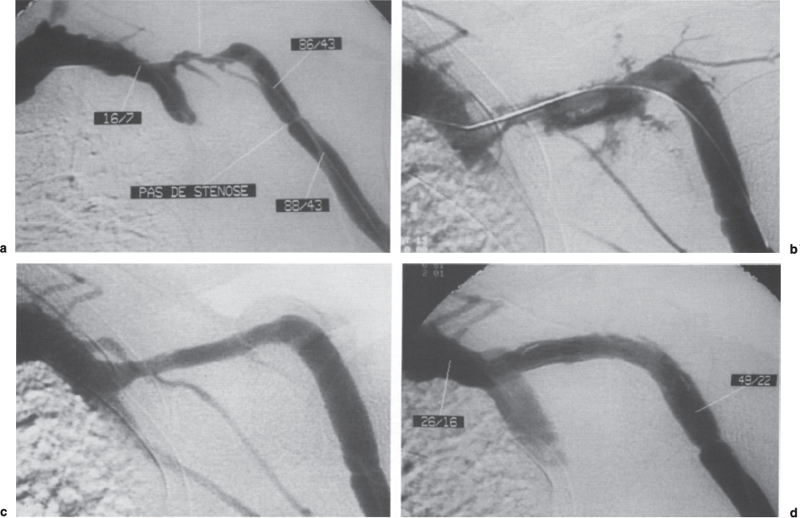
FIGURE 21–3. (a) Angiography of this brachiocephalic fistula was performed for increased compression times after dialysis. Stenosis of the final arch of the cephalic vein resulted in an 81% drop in systolic pressure [(86–16):86 = 81]. (b) Dilation to 7 mm resulted in a rupture, which was easily treated because the guidewire had been left through the lesion. (c) Balloon tamponade controlled the rupture, but there was obvious residual stenosis, probably due to the surrounding hematoma. (d) Placement of a self-expandable stent (here a Craggstent*) improved the local diameter but with a residual 47% drop in systolic pressure [(49–26):49 = 47]. The fistula thrombosed 13 months later because of restenosis immediately downstream from the stent, and it was successfully declotted percutaneously.
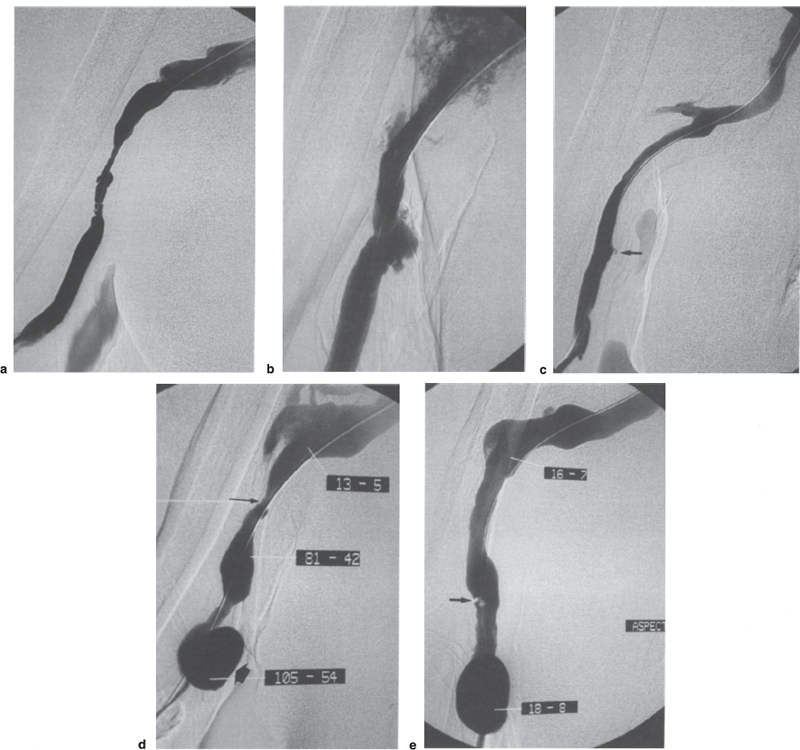
FIGURE 21–4. (a) Stenosis in the axillary outflow of such a transposed brachiobasilic fistula is very frequent. (b) Dilation to 10 mm resulted in three areas of rupture, a frequent complication in this type of fistula in this location. (c) Rupture was controlled by prolonged low-pressure tamponade with the same 10-mm balloon. Residual stenosis was due to the surrounding hematoma, but no stent was placed because the outcome is usually more favorable than in the cephalic vein. Minor residual leakage remained visible in the lower part (arrow). (d) Increasing venous pressures necessitated further angiography 5 months later. Restenosis was confirmed (thin arrow), mainly in the upper part [(81–13):81 = 84%], and the former residual leakage of the lower part had become a self-limiting pseudoaneurysm (wide arrow). (e) Simple redilation to 10 mm provided an excellent hemodynamic result, with the disappearance of any pressure gradient despite minor parietal damage (arrow). The aneurysm was asymptomatic and has been monitored clinically for 3 months.
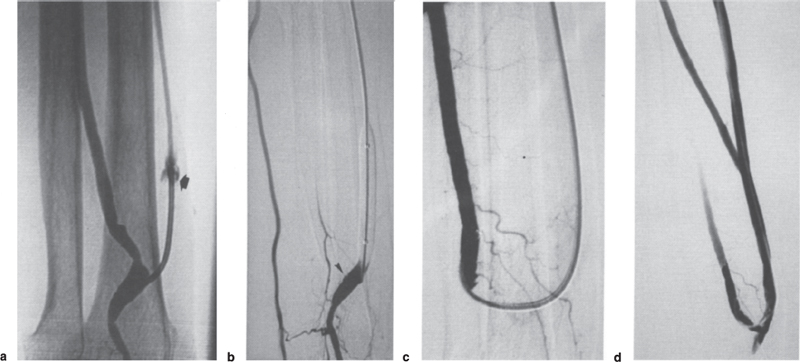
FIGURE 21–5. (a) Insufficient inflow in this radiocephalic fistula was due to an immediately postanastomotic stenosis. The stenosis was traversed using a retrograde approach with difficulty after a “wrong way,” which created small extravasation (arrow). The stenosis was so tight that the 5F catheter was occlusive. (b) The flow remained abnormally low after dilation of the anastomotic area to 7 mm, and injection through the end hole of the reinflated balloon showed that dilation of the anastomotic venous stenosis caused extrinsic occlusion of the proximal radial artery, whose anastomosis was nevertheless still recognizable as a tiny stump (arrowhead). (c) Selective catheterization of the occluded artery was nevertheless possible from the fistula and the anastomosis was dilated with a 5-mm balloon. (d) Final angiogram showed the reopening of the proximal artery, and the flow was finally excellent.
High flow is mainly a complication of upper-arm fistulae and is a contraindication for dilation because treatment of the stenosis would increase the already high flow (Fig. 21–8
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree