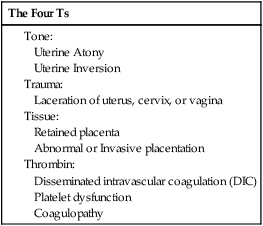
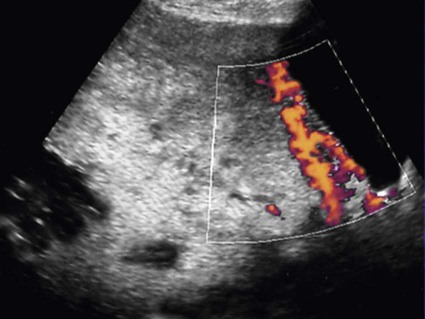
Peripartum hemorrhage (PPH) is reported as the most common maternal morbidity in developed countries and a major cause of death worldwide. Globally, postpartum hemorrhage may account for 25% of delivery-associated deaths.1 The incidence may be up to 20% of pregnancies beyond 18 weeks’ gestation. In the United States, PPH may occur in 1% to 5% of deliveries.2 The use of embolization to control pelvic hemorrhage was first described in 1972.3 As a treatment for pelvic fractures, the technique was successful and later applied to the treatment of PPH as described by Brown et al. in 1979.4 With several refinements over the years, this procedure has emerged as a safe and highly effective option for treating PPH. The etiologies for PPH can be summarized in the 4Ts mnemonic: tone, trauma, tissue, and thrombin (Table 77-1). Uterine atony is the most common cause of PPH, accounting for 70% to 80% of cases.5 This occurs when there is decreased contraction of the myometrium. The postgravid uterus is floppy or flaccid and unable to provide adequate compression for hemostasis. Risk factors for atony include multiple gestation, fetal macrosomia, and prolonged labor. Birth trauma leading to lacerations and hematomas may be the cause of significant PPH, with an incidence of about 20% in these patients.5 Injuries to the vagina, cervix, and uterus leads to hemorrhage, given the highly increased vascularity that has developed during pregnancy. Risk factors that increase the trauma of delivery include fetal macrosomia, any instrumentation (e.g., forceps, vacuum), vaginal birth after cesarean section, or episiotomy.2 A small subset of patients with birth trauma may sustain substantial injury to the uterus classified as uterine rupture. The incidence is 0.6% in patients who undergo vaginal birth after cesarean section.6 The risk for uterine rupture increases with classical cesarean incisions, short interval between pregnancy, and a history of multiple cesarean deliveries, especially in women with no previous vaginal delivery. The use of pharmaceutical induction and/or augmentation of labor can increase the rate of uterine rupture as well. On average the placental delivery occurs within 10 minutes following the fetus. The diagnosis of retained placenta is defined as the absence of placental expulsion by 30 minutes. In about 10% of patients with PPH, retained placental tissue is the primary etiology.5 The operator may attempt several manipulations to retrieve the retained tissue and/or treat the ensuing blood loss. However, in a fraction of these patients, the tissue plane between the uterine wall and the placenta cannot be identified or separated, and the diagnosis of abnormal placentation or invasive placenta should be considered. Risk factors for retained placenta include any events that may have damaged the endometrial lining before becoming pregnant, multilobed placenta, prior uterine surgery, or cesarean section delivery. The presence of invasive placenta can be life threatening. In the United States, the overall incidence is 1 in 533 deliveries, a rate that appears to be increasing and likely secondary to the increased rate of cesarean section.7 There are three different types of invasive placenta with reference to the depth of invasion: (1) placenta accreta, which adheres to the myometrium, (2) placenta increta, which invades the myometrium, and (3) placenta percreta, which penetrates the myometrium to or through the serosa. Coagulation disorders may result in PPH and may be the causal etiology in 1% of patients.5 Preexisting disease may be discovered prior to delivery and allow for preparation and appropriate prophylaxis. Examples include idiopathic thrombocytopenic purpura (ITP), thrombotic thrombocytopenic purpura (TTP), factor X deficiency, familial hypofibrinogenemia, and von Willebrand disease. Disseminated intravascular coagulation (DIC) can occur in the setting of sepsis, placental abruption, and amniotic fluid embolism. For patients with suspected PPH, ultrasound may show hematoma, retained placental fragments, or possibly abnormal placental invasion. Color flow Doppler images are especially helpful to identify the vascularity of any products of conception and demonstrate the abnormal lacunae of an invasive placenta (Fig. 77-1). The desire to maintain fertility may be a relative contraindication to pelvic arterial embolization. The pelvis has a rich and varied arterial supply, optimal for collateral arterial supply and support of the uterus following embolization.8 However, the risk for temporary or even permanent menopause post embolization exists, given the common occurrence of anastomoses between the ovarian and uterine arteries.9 Additionally, there may be increased risk for subsequent pregnancy complications such as spontaneous abortion, preterm delivery, and abnormal placentation after uterine artery embolization.9,10 Although future fertility is an important consideration for all patients who undergo pelvic embolization, in the face of ongoing hemorrhage, this may not be the clinical priority. Particle embolics such as polyvinyl alcohol (PVA), Embosphere (BioSphere Medical, South Jordan, Utah), and Embozene microspheres (CeloNova BioSciences, Peachtree City, Ga.) are another choice in the treatment of PPH. The gelatin microspheres are U.S. Food and Drug Administration (FDA) approved and used frequently for uterine artery embolization; microspheres are considered permanent embolic agents. Particles are more costly than gelatin sponges, and there is some controversy over the increased likelihood for ischemia or necrosis.11 N-butyl cyanoacrylate (NBCA) glue has also been described as an effective embolic for pelvic hemorrhage.12 The delivery of glue is more technically challenging, but it is a viable option if the use of other embolic materials is unsuccessful.
Peripartum Hemorrhage
Introduction
Pathophysiology
Imaging
Contraindications
Methods and Materials
Pelvic Arterial Embolization
Embolic Agents
Radiology Key
Fastest Radiology Insight Engine