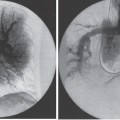
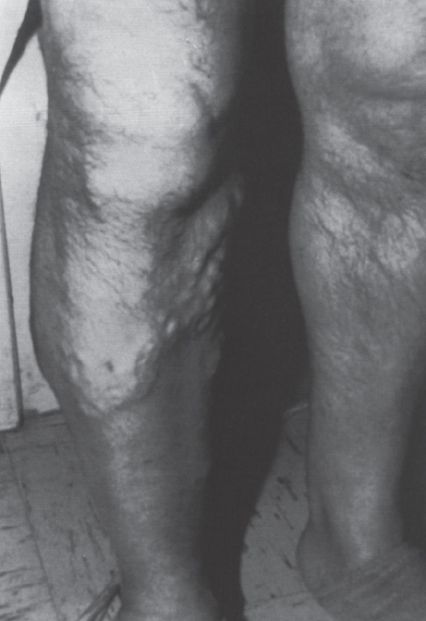
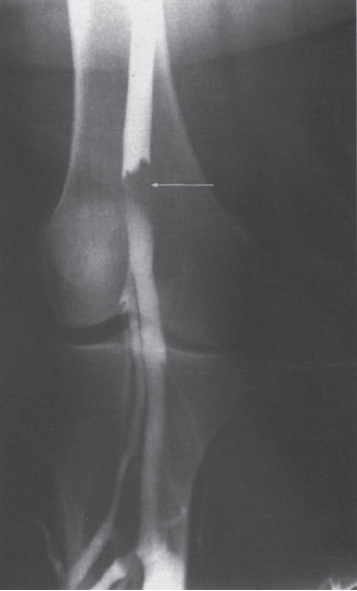
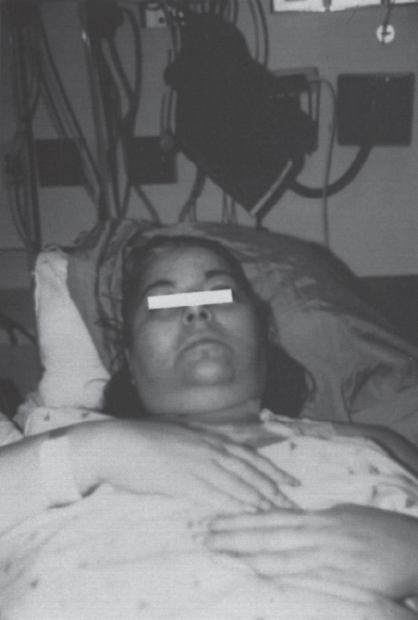
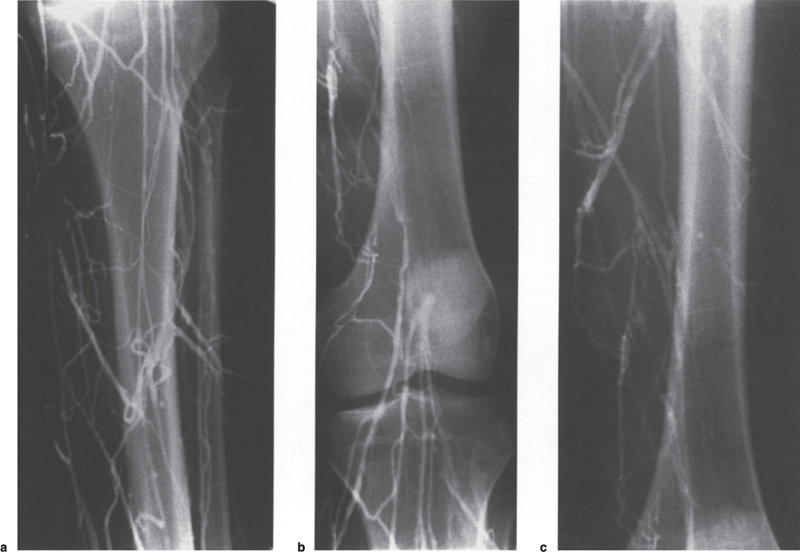
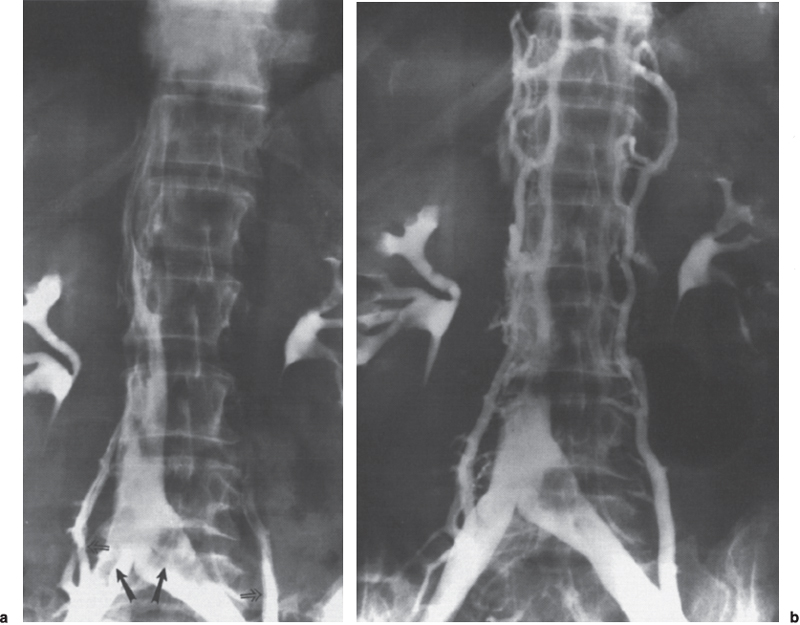
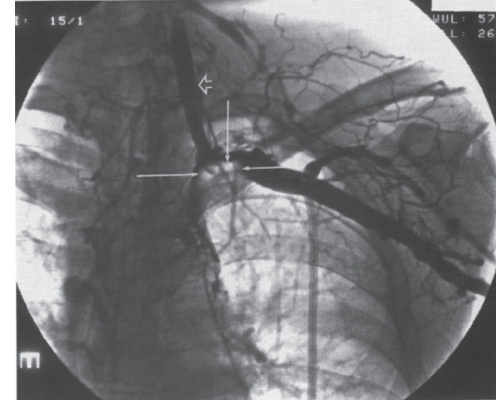
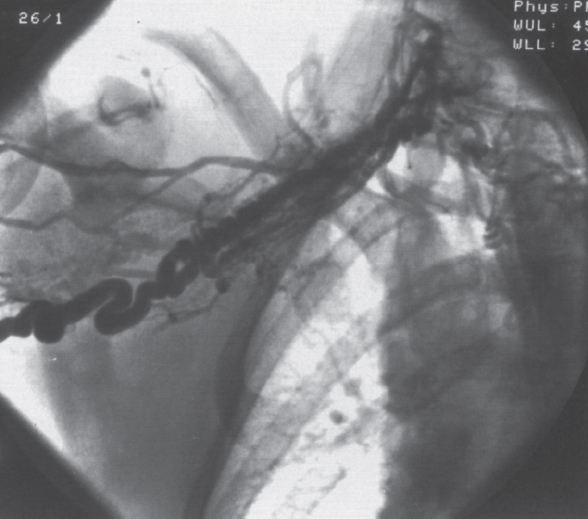
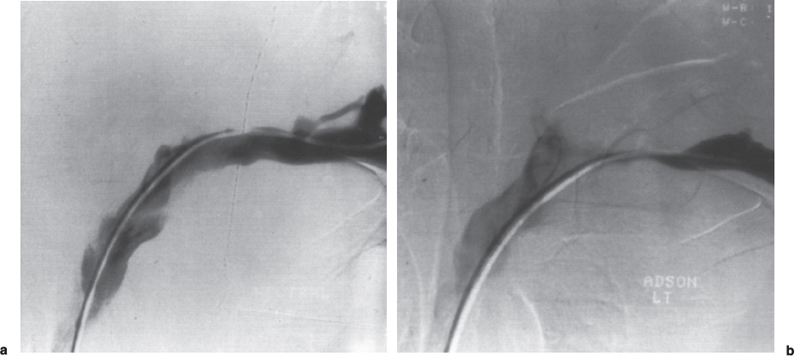
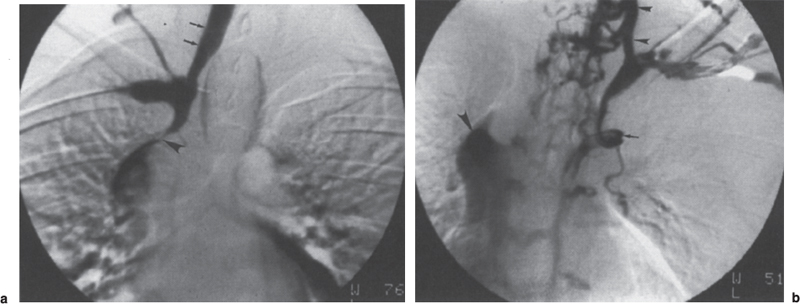
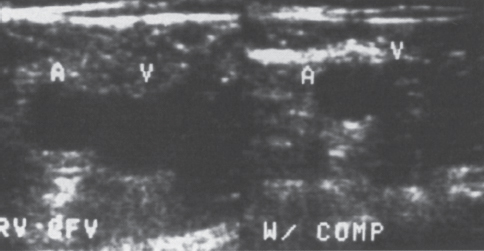
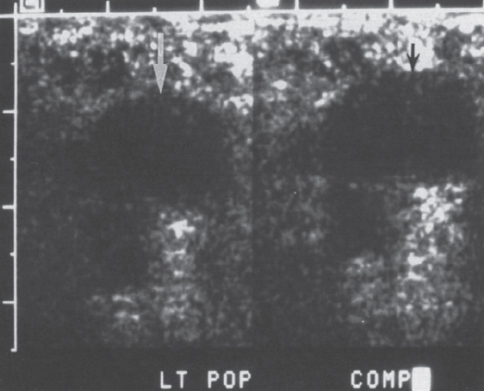
Peripheral and Central Deep Venous Thrombosis Approximately 20 million cases of lower-extremity and at least 200,000 to 600,000 cases of upper-extremity deep venous thrombosis (DVT) occur annually in the United States.1–3 The most significant consequence, pulmonary embolism, is estimated to occur in 750,000 to 900,000 of these persons, with a resultant annual mortality of 17,000 to 200,000 patients.1,4,5 During the healing phase of DVT, permanent damage can occur to the venous system, particularly the valve leaflets, as a direct result of clot retraction and resorption and venous outflow obstruction. The net result of this irreversible injury is chronic venous hypertension (Fig. 32–1). The exact incidence of the “postphlebitic syndrome” is difficult to estimate; however, in one study, only 21% of patients were asymptomatic 5 to 10 years after venographically proven DVT.6 A study performed in Michigan by Coon et al7 involving more than 9000 persons aged over 20 years demonstrated the prevalence of active or healed venous stasis ulcers to be 0.005%. Extrapolation of this figure to the general U.S. population would suggest that 500,000 persons have experienced or suffer from chronic sequelae directly related to DVT.8 The pathophysiology involved in DVT is complex; however, as early as 1860, Virchow described the triad of (1) abnormal venous flow dynamics, (2) abnormalities of the vein wall, and (3) a hypercoagulable state as playing a key role. Abnormalities in venous flow can occur as a result of external compression, trauma, intraluminal foreign bodies, decreased cardiac output, or prolonged immobilization. Vein-wall injury can result from previous DVT, trauma from central venous catheters, or from venous infusions of toxic or caustic agents (e.g., chemotherapeutic drugs, lipids). A hypercoagulable state is commonly associated with polycythemia vera rubra, antithrombin III deficiency, protein C or S deficiency, homocystinuria, malignancy, oral estrogen use, and pregnancy and the postpartum state. These are but a few examples of how conditions addressed by Virchow’s triad can occur. Piccioli, et al9 concluded from a registry of 150 consecutive patients with DVT that surgery (47%), cancer (26%), and previous thromboembolism (23%) were the main culprits in the development of DVT. Despite these findings, other studies10 concluded that at present there is no clinical case for routine preoperative or postoperative screening of surgical patients with hemostatic tests to predict or identify postoperative DVT. The most common clinical presentation in patients with DVT is sudden onset of swelling of an extremity. The extremity may be painful, red, and warm to the touch. Pain may be elicited on palpation along the course of the deep venous system. A “cord” may be felt during palpation the thrombosed vessel itself (Fig. 32–2). Fever and chills are uncommon and usually are due to an underlying problem. The swelling is due to obstruction of the venous outflow, usually related to a combination of damage to the deep valves and to thrombosis.11 The signs and symptoms of DVT are inconsistent and unreliable, however. Unilateral swelling is the most reliable physical finding in patients with DVT. The classically described Homan’s sign (pain in the calf with passive dorsiflexion of the foot) is much less reliable.11–13 In two thirds of patients, venous thrombosis is clinically silent and detected only by radiologic imaging techniques or at autopsy.13,14,15 More commonly, patients present with clinical findings suggestive of pulmonary embolism (PE) rather than DVT. There is a well-recognized association between DVT and the occurrence of pulmonary thromboembolism. According to a study from the National Institutes of Health,16 DVT causes 300,000 to 600,000 hospitalizations and 50,000 deaths yearly. Asymptomatic lower-extremity DVT has been documented in 70 to 80% of patients with objectively confirmed symptomatic PE.5,17 FIGURE 32–1. Chronic venous hypertension. Note the prominent superficial venous system secondary for venous hypertension (Photo courtesy of Anthony C. Venbrux, M.D., Johns Hopkins Hospital, Baltimore, MD.) Patients with upper-extremity DVT, in addition to limb swelling, may experience swelling of the face and neck if the thrombus extends more centrally to involve the brachiocephalic veins and superior vena cava (Fig. 32–3). Dilated collateral veins may appear on the chest, and symptoms of dyspnea and cyanosis are not uncommon. These findings, in the face of upper-extremity DVT, constitute what is termed the superior vena cava syndrome. PE from upper-extremity DVT occurs at a rate of 9 to 25%.5 These statistics lend further proof as to the insidious nature of this “benign” disease. FIGURE 32–2. Popliteal vein deep venous thrombosis in obese female. Note the filling defect with irregular margin in the popliteal vein. Phlegmasia cerulea dolens (PCD) is a rare condition of arterial insufficiency caused by total venous obstruction within at least one limb. The original description by Gregorie18 in 1938 included venous thrombosis, painful ischemia with loss of distal pulses, and purple ecchymosis. Clinical presentation includes rapid accumulation of massive limb edema with cyanotic changes beginning in the feet and toes and advancement proximally with eventual involvement of the entire limb. On physical examination, the limb is cool to the touch, tense, and indurated.19 The iliofemoral, pelvic, cross-pelvic collaterals, and distal inferior vena cava (IVC) often are involved.20 Arterial insufficiency occurs riot so much from the venous outflow obstruction; rather, the increasing interstitial tissue pressure eventually supersedes the arterial closing pressure, resulting in arterial collapse.21 The consequences of delayed treatment are significant and include venous gangrene (55%), pulmonary embolism (22%), limb amputation (50%), and death (25–32%).22–24 FIGURE 32–3. Patient with superior vena cava syndrome secondary to lung carcinoma (Photo courtesy of Anthony C. Venbrux, M.D., Johns Hopkins Hospital, Baltimore, MD.) Lower Extremity Estimates are that the lower extremity is involved in 94 to 99% of cases of DVT. Venous thromboembolism is diagnosed in about 1 % of hospitalized patients.25 Autopsy data have shown that subclinical DVT occurs in at least an additional 1 % of these patients.2,26,27 The etiology for lower-extremity DVT is shown in Table 32–1. In a study by Wheeler and Anderson,11 risk factors for DVT were evaluated in 1231 patients. Age of 40 years or older (88.5%), obesity (37.8%) (Fig. 32–2), prior history of venous thromboembolism (26%), malignancy (22.2%), bed rest for 5 days or longer (12%), and major surgery (11.7%) were identified in at least 10% of this population.25 DVT may occur in 35 to 54% of patients who undergo hip surgery, with a fatal PE rate of 0.3 to 10%.8,28–31 Following open prostatectomy, 22 to 51% of patients may develop venous thromboembolism.32–35 Patients who undergo surgery for malignant gynecologic or thoracoabdominal disease may face a postsurgical risk of DVT as high as 33 to 60%.36–38,78 Twelve percent of patients with paralyzed limbs,39 0.27 to 2% of postpartum women,40 and 20 to 40% of patients postmyocardial infarction also may experience lower-extremity DVT. Following placement of an IVC filter, localized common femoral vein trauma can result in subsequent venous thrombosis in 10 to 41% of patients, although symptomatic presentation occurs in only 3%.40 In an extensive study by Kerr et al,41 DVT was diagnosed in 1084 lower extremities in 953 symptomatic patients (14% bilateral DVT) and categorized by location: 35% of limbs demonstrated infrapopliteal thrombus with the posterior tibial vein most commonly involved (39%); 16% of limbs demonstrated thrombus within the greater or lesser saphenous vein; and 51% of limbs demonstrated thrombotic involvement of the popliteal, deep femoral, superficial, or common femoral vein (Fig. 32–4). Overall, most cases of lower-extremity DVT (symptomatic and asymptomatic) develop within or are limited to the deep veins of the calf.3,42 Forty percent resolve without treatment and 40% mature, retract, become endothelialized, or recanalize without propagation.42 The remaining 20% propagate into the popliteal, superficial femoral, and iliac veins or IVC.43 Histologic analysis has shown that thrombi are predominantly composed of fibrin and red blood cells, with platelets and leukocytes forming a variable component.44 These thrombi usually begin in areas with low flow or turbulence (nonlaminar flow), such as the valve cusp pockets or large venous sinuses of the calf.42,45,46 Propagation versus spontaneous resolution of thrombi is a complex process in which the stimulus of the inciting event is weighed against naturally occurring thrombolytic mechanisms. These mechanisms include (1) inactivation of activated coagulation factors; (2) reduction of platelet aggregation activity; (3) initiation and enhancement of thrombolysis through activation of protein C, protein S, and antithrombin III; (4) activation of the plasmin-mediated thrombolytic system; and (5) reticuloendothelial cell clearance of soluble fibrin complexes.8 Primary Idiopathic Secondary Malignancy Trousseau’s syndrome Tumor or lymph node compression Postradiation therapy Trauma Venous catheterization Inferior vena cava filter placement Hip/pelvic fracture Hypercoagulability syndrome Oral contraceptives Protein C or S deficiency Antithrombin III deficiency Polycythemia rubra vera Pregnancy and postpartum state Activated protein C resistance Anticardiolipin syndrome Thrombophlebitis Septic Contrast related Prolonged immobilization Spinal cord injury Stroke Sitting for extended periods (i.e., driving, flying) Low venous flow states Myocardial infarction Congestive heart failure Varicose veins Rapid dehydration Anatomic abnormalities Obesity Prior history of thromboembolism Age (>40 yr) Heparin-induced thrombocytopenia FIGURE 32–4. (a) Lower leg, (b) knee level, and (c) thigh level views obtained during this venogram demonstrate thrombosis of the entire deep venous system. None of the veins of the deep venous system is visualized; however, extensive collateralization is present throughout the leg, affording compromised venous drainage. Asymptomatic PE can occur in up to 50% of patients who present with DVT47–49 and is more common when larger (popliteal, superficial femoral veins) rather than smaller (infrapopliteal) veins are involved. Seventy percent of patients with confirmed PE demonstrate asymptomatic DVT, typically involving the larger veins. Before the introduction of anticoagulant therapy, the mortality rate for PE was 10% in patients with recognized DVT,50 and the recurrent rate of PE was about 50%. Untreated PE carried a 25% mortality rate.51–53 Inferior Vena Cava The etiologies for lower extremity DVT shown in Table 32–1 also apply to the JVC. Thrombosis of the IVC can occur as a direct result of extension of lower extremity DVT; however, this is atypical unless bilateral disease or other factors are also present. Malignancy and coagulopathic disorders, such as polycythemia rubra vera and antithrombin III deficiency, can increase the risk of IVC thrombosis de novo or as a consequence of lower-extremity DVT (Fig. 32–5). Anomalies of the IVC, such as webs and stenoses, also can result in thrombosis. Iatrogenic foreign bodies, such as an IVC filter, can cause thrombosis in 5 to 80% of patients, depending on the design of the filter, the length of time the filter is in place, and the patient’s overall health. FIGURE 32–5. Patient with leiomyosarcoma of the duodenum and liver presented with bilateral lower-extremity edema, (a) Early phase of an inferior vena cavogram demonstrates filling defects in both common iliac veins (arrows) with extension into the lower portion of the inferior vena cava. Note that early filling of both ascending lumbar veins (open arrows), (b) Late phase from the inferior vena cavogram demonstrates occlusion of the inferior vena cava with collateralization from the ascending lumbar veins to the paravertebral veins. The IVC may be compressed extrinsically by a mass such as a tumor or lymph nodes (Fig. 32–5). Tumors such as hepatoma, renal cell carcinoma, and leiomyosarcoma of the IVC may cause intrinsic obstruction of the IVC. Pregnancy may result in IVC compression from the enlarged gravid uterus. Upper Extremity Deep venous thrombosis of the upper extremity accounts for 1 to 4% of all symptomatic thromboembolic events.54–58 Proposed factors that could explain the disproportionately small number of cases of upper-extremity versus lower-extremity DVT include the relative absence of thrombogenic valves (particularly in the great vessels of the chest), decreased hydrostatic forces, improved gravitational effects resulting in decreased stasis, higher rates of blood flow, less frequent immobility, and increased (up to four times) fibrinolytic activity in the upper-versus lower-extremity venous endothelium.56,59–62 The etiology of upper-extremity DVT is shown in Table 32–2. The rapidly increasing use of central venous catheters has resulted in a greater recognition of this entity.56–58 At least one recent study reported that the cause of upper extremity DVT is fairly evenly divided among intravenous catheters (32%), anatomic abnormalities (45%), and carcinoma with postoperative radiation therapy (22.5%).56 We believe these figures strongly reflect patient population and referral patterns. At our institution, by far the vast majority of patients evaluated venographically for upper-extremity DVT have indwelling central venous catheters. Primary DVT Idiopathic Effort thrombosis (Päget-Schroetter syndrome) Secondary DVT Catheter/pacemaker wire related Malignancy Trousseau’s syndrome Tumor or lymph node compression Postradiation therapy Trauma Venous catheterization Repeated venipuncture Blunt or penetrating Clavicular fracture Axillary surgery Hypercoagulability syndromes Antithrombin III deficiency Polycythemia rubra vera Protein C or S deficiency Oral contraceptives Benign fibrosing mediastinitis Tuberculosis Histoplasmosis Thrombophlebitis Septic Contrast related Infusaid related (i.e., chemotherapeutic agents, hyperalimentation formulas) Central venous stenosis secondary to upper extremity dialysis fistula Rapid dehydration Congestive heart failure Anatomic abnormalities DVT, deep venous thrombosis. Central Venous and Tunneled Catheters The use of upper-extremity central venous catheters has been increasing steadily during the past decade. Hill and Berry56 reported a 560% increase in the use of such catheters from 1982 to 1987. The incidence of symptomatic subclavian vein thrombosis in this patient group may be as high as 50%. Some of this variability can be accounted for based on critical factors pertaining to catheter-related DVT. First, it has been demonstrated that the incidence of catheter-related DVT is increased with increasing catheter size.58 Although subclavian vein DVT may be seen in an average of about 30% of patients with catheters (Fig. 32–6), it occurs in only 0.18% of patients who have pacemaker leads inserted.55 Second, the number of indwelling catheter days also plays a factor, and thrombosis rates are increased proportionally as the catheter’s life increases. Third, catheter material also can play a role; silicone elastomer (Silastic) catheters have been shown to be less thrombogenic that polyurethane and polyvinyl chloride catheters.58,62,63 Siliconized polyethylene catheters are more thrombogenic than Teflon or heparinized polyethylene catheters.64 In addition, patient health factors can play a significant role in the development of catheter-related DVT. Sepsis, decreased cardiac output, a hypercoagulable state, carcinoma, and radiation therapy contribute to subclavian vein thrombosis.56 The composition of the infusate can play a significant role, and thrombosis rates have been correlated with amino acid composition, pH, and osmolality.58,63–65 The incidence of catheter-related upper-extremity DVT with embolization to the lungs is 1.5 to 6.4%, with a mortality rate of 0.75 to 4.3%.64,66 FIGURE 32–6. Subclavian vein thrombus in patient with double-lumen catheter. A large filling defect (arrows) is present in the left subclavian vein at the insertion site of the double-lumen catheter. Note the retrograde filling of the left internal jugular vein (open arrow). Effort Thrombosis Effort thrombosis, or Päget-Schroetter syndrome, is defined as upper extremity DVT in a healthy person without a precipitating cause, other than recent (strenuous) activity involving the arm. Most patients are young men aged under 40 years; however, the syndrome can occur in active women. Precipitating events may be absent. Symptoms consist of upper-extremity pain, swelling, weakness, and limited motion.67 Dilated superficial veins and a palpable axillary “cord” also may be noted. The right arm appears to be involved more frequently than the left.68–71 The apparent etiology is compression of the subclavian vein between the costocoracoid ligament and first rib, subclavius muscle and first rib, or clavicle and first rib. In some cases, hyperabduction of the arm may simply result in stretching of the vein to the point of inducing a traumatic endothelial injury (Fig. 32–7).69 Diagnosis is obtained most commonly by venographic evaluation (Fig. 32–8a,b). Despite the invasive nature, venograms are readily available in most hospitals, are easy to perform, have minimal associated morbidity and mortality, and clearly define the point of obstruction and extent of the collateral network. Color-flow Doppler ultrasound and duplex techniques also can be used. These techniques have demonstrated both a high sensitivity and specificity for the diagnosis of subclavian thrombosis.72–74 FIGURE 32–7. Päget-Schroetter syndrome in a 16-year-old girl with a history of vigorous upper-extremity exercise followed by painful swelling of the right arm over the ensuing 3 to 4 days. Note the extensive collateral system including a relatively large single vein and innumerable tiny channels that developed secondary to thrombosis of the main veins. Patient was treated with a peripheral streptokinase infusion, which resulted in some improvement; however, therapy was stopped secondary to a lower gastrointestinal hemorrhage. Patient was stabilized, converted to warfarin sodium, and discharged with a prothrombin time ratio of 1.6 times control. Surgery traditionally has been used as a primary treatment modality if underlying venous disease is discovered (e.g., venous stricture, venous compression from clavicle). In the absence of these findings, however, treatment has tended to be more conservative, ranging from rest and limb elevation75 to catheter-directed thrombolysis and venous angioplasty.67,70 At the very least, anticoagulant therapy should be used during the acute phase to prevent extension of the clot into collateral vessels and the great veins of the chest.76,77 More recent efforts focused on the potential benefits of thrombolytic therapy. In a study by Hill and Berry,56 nine patients with effort thrombosis were treated with either urokinase or streptokinase. Two patients required follow-up surgery to correct a persistent narrowing of the subclavian vein. All nine patients received short-term anticoagulant therapy after hospital discharge. Three (33%) recurrences of vessel thrombosis occurred, all in patients with an underlying anatomic narrowing of the subclavian vein. In a study by Grassi and Bettmann67 in which 11 patients experienced 19 effort thrombosis-related events, 18 events were treated with heparin, seven also were treated with urokinase, and four patients had percutaneous transluminal angioplasty performed in conjunction with heparin or urokinase therapy. Four patients also had a surgical thrombectomy or bypass. None of the treatments led to a predictable outcome or long-term venous patency. Rather, venous patency tended to be transient, and symptomatic relief was no greater in persons who had a successful recanalization procedure compared with those who spontaneously developed adequate collaterals. Thus, the best treatment modality for patients with effort thrombosis remains unclearly defined. FIGURE 32–8. (a) Digital subtraction venography of the subclavian vein in the neutral position. (b) Digital subtraction venography with the patient performing the Adson’s maneuver. The stenosis at the subclavian vein is accentuated in this position. (Photos courtesy of Anthony C. Venbrux, M.D., Johns Hopkins Hospital, Baltimore, M.D.) Other Etiologies of Upper-extremity DVT Upper-extremity DVT may result from direct compression of the subclavian vein by one or more of many associated anatomic structures (thoracic outlet compression syndrome), including the scalenus anterior muscle, first rib, cervical rib, anteriorally positioned phrenic nerve, pectoralis minor muscle, coracoid ligament, sternocostoclavicular hyperostosis, aberrant transverse cervical artery, malunited clavicular fracture, or persistent axillopectoral muscle.71,78–80 Malignancy may directly result in up to 24% of the reported cases of upper-extremity DVT.56,58,81,82 Carcinoma with postoperative radiation therapy resulted in 22.5% of the cases of upper-extremity DVT reported by Hill and Berry.56 Trousseau’s syndrome resulting in upper-extremity DVT is uncommon but reported to occur in 2% of patients who experience peripheral thrombosis.76,83 Oral contraceptives have been reported as the cause of upper-extremity DVT in case reports.84,85 Hematologic disorders resulting in a hypercoagulable syndrome, for example, polycythemia vera, protein C and S deficiency, and antithrombin III deficiency, can result in upper-extremity DVT. Congestive heart failure also has been reported as a cause.86 Superior Vena Cava The etiologies for upper-extremity DVT shown in Table 32–2 also apply to thrombosis of the superior vena cava (SVC). As in the pelvis, thrombosis of the SVC usually does not occur with unilateral subclavian vein thrombosis because of the inflow from the contralateral extremity. Bilateral subclavian vein thrombosis, however, can result in thrombosis of the SVC, particularly if other factors, such as the presence of a catheter, a hypercoagulability syndrome, or septic thrombophlebitis, are present. Isolated SVC thrombosis is predominantiy seen in cases of malignancy that involve the mediastinum, such as lung carcinoma and lymphoma (Fig. 32–9). Benign causes of mediastinitis, such as tuberculosis and histoplasmosis, also may result in isolated superior vena cava obstruction. FIGURE 32–9. Patient with lung carcinoma and superior vena cava syndrome, (a) Digital subtraction image from a right upper-extremity venogram demonstrates occlusion of the right brachiocephalic vein (arrowhead) and extensive retrograde filling of the right internal jugular vein (arrows), (b) Digital subtraction image from a left upper extremity venogram demonstrates occlusion of the left brachicephalic vein with filling of the left superior intercostal vein (arrow) and retrograde filling of the left internal jugular vein (small arrowheads). Both systems drain through the paravertebral plexus into the azygos vein (large arrowhead), thus affording upper extremity venous return to the heart. Noninvasive Modalities: Duplex, Compression Doppler, and Color-flow Techniques Lower Extremity This technique has been evaluated in the suprapopliteal veins by numerous investigators; sensitivities and specificities ranged from from 87 to 96% and 89 to 100%, respectively.87–92 In two studies in which the infrapopliteal veins also were evaluated, duplex-Doppler ultrasound correlated with the venographic findings in 63 to 89% of cases.89,91 The diagnosis of DVT is made by sonographic real-time evaluation of the common femoral to popliteal veins using a 5- to 10-MHz transducer. Some authors also image the deep femoral and infrapopliteal veins.41 Normal veins have a classic venous Doppler signal and can be compressed by the transducer with obliteration of the lumen and cessation of flow (Fig. 32–10). In addition, phasicity, or changes in the Doppler signal, can be detected easily during the respiratory cycle or with forced inspiration or expiration. Manual squeezing of the patient’s calf or contraction of the muscles of the leg results in augmentation of the venous blood flow. Criteria for a positive examination (i.e., for a diagnosis of DVT) include (1) noncompressibility of the vessel (Fig. 32–11); (2) little or no change in the caliber of the vessel during Valsalva maneuver, coughing, or sniff test; (3) absent Doppler signal (Fig. 32–12); (4) visible thrombus (hypoechoic to hyperechoic intraluminal mass) within the vein (Fig. 32–13); and (5) an absence of phasicity, spontaneity, or augmentation.87,92,93 Potential limitations of ultrasound are low sensitivity for calf thrombosis as a result of frequent variant anatomy and the small caliber of the veins. In addition, it is difficult to detect isolated thrombi at the level of the iliac vein and the superficial femoral vein within the adductor canal. When evaluating for recurrent DVT, noninvasive tests are useful only when the test becomes normal during follow-up of the first episode. Contrast venography may be a better diagnostic tool for recurrent DVT if the normalization is incomplete by noninvasive methods.94 FIGURE 32–10. Compression ultrasound in a normal patient. The lumen of the common femoral artery (A) and vein (V) are easily visualized on the left image. On the right image, compression has been applied, which obliterates the lumen of the vein. Note that the arterial lumen is not compressible. FIGURE 32–11. Compression ultrasound in patient with popliteal deep venous thrombosis (DVT). The image on the left clearly demonstrates the lumen (arrow) of the popliteal vein. The right image, obtained following application of compression with the transducer, fails to show obliteration or any change in appearance of the lumen (arrow) of the popliteal vein. This lack of compressibility is consistent with DVT. Chronic DVT can be diagnosed based on the presence of (1) thickened vein walls with hyperechoic intraluminal projections (Fig. 32–14), (2) the presence of collateral vessels, (3) noncompressibility of patent veins, and (4) little or no change in venous caliber with alterations in flow.89,93,95

 Presentation
Presentation
 Phlegmasia Cerulea Dolens
Phlegmasia Cerulea Dolens
 Etiology of Deep Venous Thrombosis
Etiology of Deep Venous Thrombosis
 Diagnostic Evaluation of DVT
Diagnostic Evaluation of DVT
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree