Jose Luiz Orlando • Francisco Ramos • Bruno C. Odisio
Peripheral vascular malformations arise as a result of a focal vascular differentiation embryologic failure leading to an abnormal development of the vascular system. They are responsible for important functional and aesthetic changes that often impact on the individual’s daily routine. Diagnosis and treatment of this condition is still considered challenging in view of the various presentations and complexity levels of lesions.
CLASSIFICATION AND DESCRIPTION
Mulliken and Glowacki1,2 described in 1982 a useful classification system, which gained acceptance by the scientific community based on the histologic findings, the flow characteristics, and the clinical aspects of the vascular anomalies. According to this classification, vascular anomalies fall into two major categories: hemangiomas and vascular malformations.1,2 Hemangiomas are not included in the scope of the this chapter and therefore will not be discussed.
Vascular malformations arise from dysplastic vascular channels. These channels, generally presented at birth, will grow in proportion to the development of the individual and will never suffer involution.1,3 Although congenital, in approximately 10% of patients, the lesions cannot be identified at birth, appearing later triggered by hormonal stimuli during adolescence and gestation or exacerbating their symptoms after infection, thrombosis, or local trauma.4 The vascular malformations were initially classified according to the prevalence of the vascular channels in five different forms: venous, lymphatic, capillary, arterial, or combined. In 1993, Jackson et al.,5 based on the classification system by Mulliken and Glowacki,1,2 reclassified vascular malformations considering its hemodynamic characteristics, dividing them into low- and high-flow lesions. The low-flow lesions are classified as venous malformation, lymphatic capillaries, or venolymphatic forms (capillary venous and the lymphatic–venous capillary lesions); the high-flow lesions are divided in arteriovenous malformations (AVMs) and arteriovenous fistulas.6 In 1996, this classification system was adopted and expanded by the International Society for the Study of Vascular Anomalies and is currently widely used.7
PATHOPHYSIOLOGY
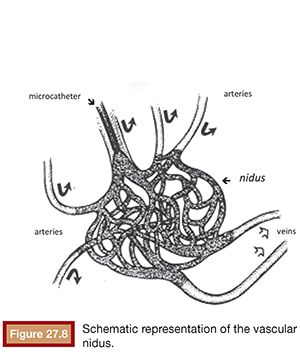
The most accepted theory for the origin of vascular malformations is that they are caused by a total or partial agenesis of the capillary bed of a given territory. This agenesis would be associated with primitive persistent arteriovenous communications that constitute the AVM nidus.8 The AVMs are basically characterized by presenting abnormal communications between the arterial and venous system, without the interposition of the capillary network. These shunts are, in most cases, multiple and configured as a conglomerate of vessels known as vascular nidus.9 The advent of digital subtraction selective angiography along with the development of microcatheterization techniques allowed the angioarchitecture of these lesions to be identified with greater precision. The angioarchitecture is divided into three distinct segments: nourishing artery(ies), a nidus, and the draining vein(s).10
The nourishing arteries can be classified as direct or indirect branches and can present as single or multiple branches. The direct branches directly supply the region of the vascular nidus and, in general, have a large diameter. The indirect branches supply the nidus and the surrounding tissues and may have blood flow opposite to its natural direction through anastomoses with neighboring arteries, where other arterial segments participate in the nutrition of the tissue. In this case, the preexisting arterioarterial anastomosis increases their caliber and promotes the passage of blood flow in a reversed direction. This compensatory phenomenon, characterized by the recruitment of collateral vessels to reconstruct the arterial supply distal to the AVM, is usually present in high-flow AVMs.11
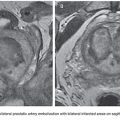
The vascular nidus represents the core of the AVM and is interposed between the distal segment of the feeding artery(ies) and the proximal segment of the draining vein(s).12 These arteriovenous communications are called shunts and are responsible for the secondary angiopathy induced by the increased blood flow. The nidus is a complex vascular structure and can be presented in three distinct patterns (Fig. 27.1):
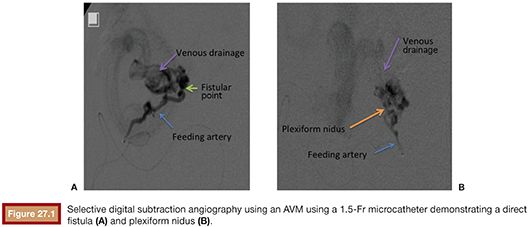
• Plexiform: composed of a tangle of arteriolar and venular structures with a coiled and wrapped aspect. In this pattern, the supplying arteries ends in a cluster of multiple vessels with arteriovenous communications in which one or multiple channels emerge as the venous drainage.
• Fistulas: characterized by the presence of communication between artery and vein called arteriovenous fistulas. In this case, one or multiple arteries of anomalous path starting from a truncal artery empties into the venous system directly or in a venous lake located, for example, in the compartment muscle or subcutaneous tissue.
• Mixed pattern: a combination of both patterns mentioned earlier, with a predominance of one over the other. The nidus may also be constituted by one or more compartments.
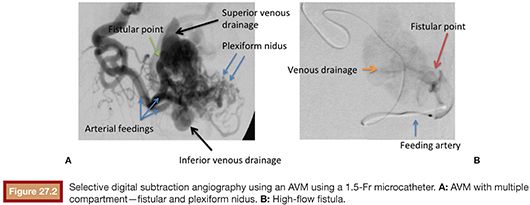
The AVM draining veins corresponds mostly to the topography of the lesion and can be classified as deep or shallow. Drainage can occur from multiple compartments to a single main draining vein or smaller caliber accessory veins. Additionally, a single drainage vein from the AVM may branch into other veins, which can be confused with multiple draining veins (Fig. 27.2).
CLINICAL EVALUATION
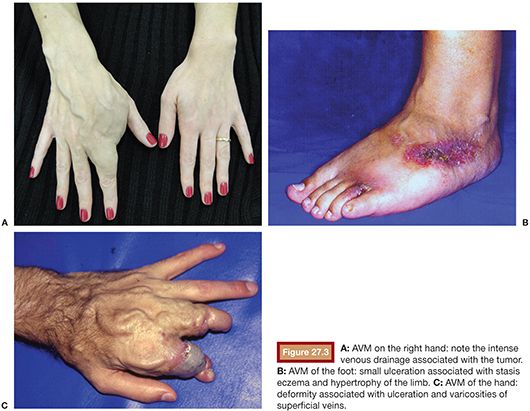
Vascular malformations can occur anywhere in the body and can have either a localized or extensive distribution pattern. Commonly, its diagnosis is the result of a clinical history and detailed physical examination. The clinical presentation of peripheral AVMs is diverse and can range from asymptomatic to disabling pain cases. The presence of pain may be related to the presence of mass effect, varicose veins, thrombophlebitis, bone erosion, and mixed-origin ulcers caused by venous hypertension due to ischemia caused by preferential blood flow to the vascular malformation in detriment of the normal tissues.1,13
The lesions on the extremities are characterized by the presence of pulsatile tumor of firm consistency and slightly compressible, with thrill or murmur that result from turbulent blood flow.14 Other characteristic findings of these injuries are a wide arterial pulse and the presence of a prominent proximal venous drainage, generally elongated and tortuous. Additionally, changes may occur secondary to the ischemia and the venous hypertension such as edema, skin pigmentation, stasis eczema, ulcers, and gangrene13,15 (Fig. 27.3). The hypertrophy of the involved extremity is a frequent finding and may be evident only in late childhood.16,17 Pelvic AVMs are characterized by an increased soft tissue mass and can cause severe pain, pelvic congestion, sexual dysfunction, and hemorrhage.18–20 In the presence of a large number of high-flow fistulae, congestive heart failure can occur in varying degrees as a result of cardiac overload.21
IMAGING EVALUATION
The use of different imaging modalities is an essential tool for differentiating between the different types of vascular malformations and between these and soft tissue tumors, assessing the exact extension of the lesions, and correlating the findings with other anatomical structures involved. The most common imaging modalities used in the diagnosis of vascular abnormalities are the ultrasonography (US), magnetic resonance imaging (MRI), computed tomography (CT), and angiography.
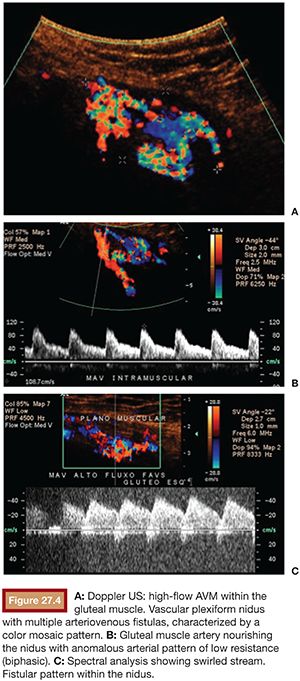
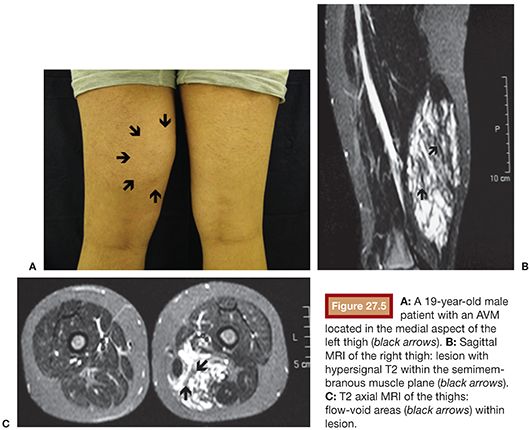
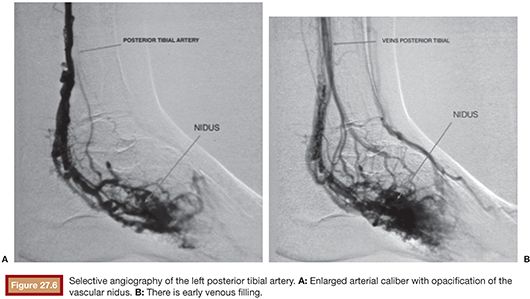
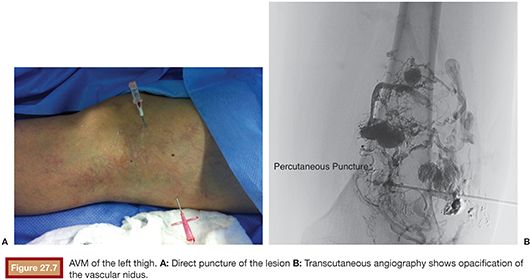
US is considered the imaging modality of choice for the initial evaluation of patients with soft tissue lesions of possible vascular origin.22,23 The US Doppler allows characterizing the morphologic and hemodynamic aspects of the vascular malformations. The color mapping allows distinguishing the afferent arteries constituted of elongated and tortuous vessels, the nidus that is characterized by a conglomerate of vessels in the core of the lesion, and the draining veins. The hemodynamic changes in high-flow vascular malformations are characterized by the presence of a biphasic pattern with high systolic and diastolic velocities, indicating low resistance due to the presence of shunts. The nidus is recognized by the presence of high-velocity turbulent flow with a mosaic of colors. The venous segment of the malformation presents with a high-flow monophasic pattern that might be associated with some pulsatile pattern (Fig. 27.4). The MRI allows for assessment of the extent of the injury and its relationship to adjacent anatomical structures. It is also useful in differentiating between lesions of high and low flow.3,24 AVMs are characterized as areas of absence of signal (flow-void) on T1 and T2 weighted sequences corresponding to the nurturing arteries and the nidus15,25 (Fig. 27.5). MRI can also help in the differential diagnosis of tumors. Noteworthy are the proliferative hemangiomas that, despite showing hyperintense signal on T2 sequences and intermediate signal on T1 and areas of flow voids, has a well-defined and lobulated contours.3,23,26 Other tumors such as sarcomas, neuroblastomas, hemangiopericytomas, fibrosarcomas, and rhabdomyosarcomas exhibit features of tissue invasion that can be associated with perilesional edema.23,27–29 CT provides limited information regarding the extent of the lesion, generally underestimating its size and its flow characteristics when compared to MRI. The use of ionizing radiation is also another disadvantage associated with this method. CT can be useful in the differential diagnosis with soft tissue tumors to evaluate its mass effect and provide more detailed evaluation of changes in the adjacent structures such as bone erosions, periosteal reaction, pathologic fractures, and presence of phleboliths. The phlebolith lesions are characteristic of venous malformations.30 The use of angiography involves the selective catheterization of all pedicles involved for a complete study of the extension of the lesion and its flow characteristics. The angiographic findings of AVMs include feeding artery(ies) with increased caliber and tortuous path, a conglomerate of arteries and veins (nidus), and an early venous filling segment characterized by elongated and large-caliber veins12,31 (Fig. 27.6). An alternative access by direct puncture of the nidus transcutaneously allows for evaluation of extension and flow characteristics31,32 (Fig. 27.7).
TREATMENT
The treatment of AVMs is still a challenge for surgeons and interventional radiologists.33,34 Surgical treatment is usually associated with some technical difficulties due to the absence of a cleavage plane between the lesion and surrounding tissue, bleeding, and inaccessible location. Surgical ligation of the nourishing arteries is ineffective and often results in recurrence of the lesion by the recruitment of numerous arterial and venous branches.17,18,20,35–38 The introduction of selective catheterization techniques created the possibility of implementing the treatment of vascular malformations through embolization. The evolution and development of new products and the introduction of microcatheters with smaller diameters allows greater selectivity of feeding vessels, making embolization the treatment of choice for vascular AVMs.15,19,39 For a proper treatment planning, it is essential to perform a diagnostic angiography for the study of the vascular anatomy of the lesion and its hemodynamic pattern.21,40 The goal of AVM embolization is the occlusion of the feeding vessels of the vascular nidus, avoiding areas of nontarget embolization (Fig. 27.8). The choice of the embolic material should be based on the lesion’s angioarchitecture, size, length, number of involved vessels, flow characteristics, and pattern of venous drainage.12,41 The limitations to a satisfactory embolization include the presence of elongated and tortuous branches and aneurysms that hinder the progression of the catheter (Figs. 27.9 and 27.10) and the presence of indirect branches or anastomoses nourishing the malformation that prevent the placement of the microcatheter within the nidus (Fig. 27.11). Additionally, these indirect branches may also supply adjacent normal tissues, increasing the risk of nontarget embolization. Most AVMs present both types of nurturing branches, and success of treatment depends on the ability to select the vascular nidus with the microcatheter.12
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree