Peritoneal Cavity
Anatomy
The various recesses and spaces of the peritoneal cavity are easiest to recognize on CT when ascites is present. Identifying the precise compartment that an abnormality is in goes a long way toward identifying the nature of the abnormality and deciding on a plan for intervention. The peritoneum is a thin membrane that produces serous fluid, which lubricates the abdominal and pelvic cavity. Parietal peritoneum lines the abdominal wall and covers the retroperitoneum. Visceral peritoneum covers organs and bowel. Whereas all the spaces of the peritoneal cavity potentially communicate with one another, diseases, such as abscesses, tend to loculate within one or more specific locations. The right subphrenic space communicates around the liver with the anterior subhepatic and posterior subhepatic space (Morison pouch). The left subphrenic space communicates freely with the left subhepatic space. The right and left subphrenic spaces are separated by the falciform ligament and do not communicate directly. The lesser sac is the isolated peritoneal compartment between the stomach and the pancreas. It communicates with the rest of the peritoneal cavity (greater sac) through the small opening of the foramen of Winslow.
The right subphrenic and subhepatic spaces communicate freely with the pelvic peritoneal cavity by means of the right paracolic gutter. The phrenicocolic ligament prevents free communication between the left subphrenic/subhepatic spaces and the left paracolic gutter. Free fluid, blood, infection, and peritoneal metastases commonly settle in the pelvis because the pelvis is the most dependent portion of the peritoneal cavity, and the pelvic recesses communicate freely with both sides of the abdomen.
The small bowel mesentery is a double layer of peritoneum that suspends the jejunum and ileum and contains branches of the superior mesenteric artery and vein, as well as mesenteric lymph nodes. The mesentery extends like a fan obliquely across the abdomen from the ligament of Treitz in the left upper quadrant to the region of the right sacroiliac joint. Disease originating from above the ligament is directed toward the right lower quadrant. Disease originating from below the ligament has open access to the pelvis.
The greater omentum is a double layer of peritoneum that hangs from the greater curvature of the stomach and descends in front of the abdominal viscera. The greater omentum encloses fat and a few blood vessels. It serves as fertile ground for implantation of peritoneal metastases.
Fluid in the Peritoneal Cavity
Fluid in the peritoneal cavity originates from many different sources and differs greatly in composition. Ascites refers to accumulation of serous fluid within the peritoneal cavity and results from cirrhosis, hypoproteinemia, congestive heart failure, or venous obstruction. Exudative ascites is associated with inflammatory processes such as pancreatitis, peritonitis, and bowel perforation. Neoplastic ascites is caused by intraperitoneal tumor. Chylous ascites is due to obstruction of or traumatic injury to the thoracic duct or cisterna chyli. Urine and bile may spread through the peritoneal cavity owing to obstruction of or injury to the urinary or biliary tracts. Hemoperitoneum is an important sign of abdominal injury in blunt trauma. When the anatomy of the peritoneal cavity is known, recognition of fluid density within its recesses on CT is easy. Paracentesis is required for precise differentiation of the exact type of fluid present in the peritoneal cavity. However, CT can offer some clues:
- •
Free intraperitoneal fluid occupies and distends the recesses of the peritoneal cavity. Bowel loops tend to float to the central abdomen. The diaphragm may be elevated by a large volume of ascites.
- •
Serous ascites has an attenuation value near that of water (-10 to 15 Hounsfield units [HU]) and tends to accumulate in the greater peritoneal space, sparing the lesser sac.
- •
Hemoperitoneum has a higher attenuation value, averaging 45 HU, and is usually above 30 HU. Blood tends to accumulate in greatest amount about the site of hemorrhage. The presence of higher-attenuation clots in the fluid is a clue to the fluid being blood.
- •
Exudative ascites due to pancreatitis tends to preferentially accumulate within the lesser sac. Exudative and neoplastic ascites have intermediate attenuation values that overlap those of serous ascites and blood. With peritonitis ( Fig. 9.1 ) the peritoneum appears thickened and enhances following intravenous contrast medium administration.

FIG. 9.1
Peritonitis and ascites.
Ascites (a) resulting from pancreatitis occupies and distends peritoneal recesses. Small bowel loops float within the fluid suspended on fat-filled mesentery (arrow) . The parietal peritoneum (arrowhead) is thickened and is enhanced following intravenous contrast medium administration, indicating peritoneal inflammation.
- •
Loculations of peritoneal fluid due to benign or malignant adhesions may simulate cystic abdominal masses. Tense loculated ascites may accumulate in confined spaces such as the lesser sac and compress and displace bowel loops. Loculated ascites, however, tends to conform to the general shape of the recesses it occupies. Cystic masses make their own space, cause greater displacement of adjacent structures, and have more varied internal consistency.
- •
Pseudomyxoma peritonei is an unusual complication of mucocele of the appendix or of mucinous cystadenocarcinoma manifested by filling of the peritoneal cavity with gelatinous mucin. The mucinous fluid is typically loculated and causes scalloping and mass effect on the liver and involved bowel ( Fig. 9.2 ). Septations, mottled densities, and calcification within the fluid may be seen on CT. Soft-tissue peritoneal implants are sometimes apparent.

FIG. 9.2
Pseudomyxoma peritonei.
High-attenuation gelatinous ascites (a) is loculated in peritoneal recesses and causes scalloping and mass effect on adjacent organs. The cause was mucinous adenocarcinoma of the stomach metastatic to the peritoneum.
Free Air in the Peritoneal Cavity
Free air within the peritoneal cavity is an important sign of a perforated intestinal tract but may be surprisingly difficult to recognize on CT when the volume of pneumoperitoneum is small:
- •
The diagnosis is based on recognizing that the air is outside the bowel lumen ( Fig. 9.3 ). Images should be routinely examined at “lung windows” (window level -400 to -600 HU; window width 1000–2000 HU) for free intraperitoneal air. Free intraperitoneal air is easiest to recognize anterior to the liver and in nondependent recesses that do not contain bowel. The very thin wall of distended bowel may be difficult to appreciate. A clue is that the air within bowel appears confined, whereas free intraperitoneal air is not confined. Rolling the patient into a decubitus position and rescanning will assist in interpretation of difficult cases.

FIG. 9.3
Pneumoperitoneum.
Small pockets of free intraperitoneal air are recognized on an abdominal CT image viewed on lung windows by the characteristic triangular and linear appearance (red arrowheads) between bowel loops in the nondependent areas of the abdomen. Note the more rounded appearance of air within bowel confined by the thin bowel wall (yellow arrows) .
- •
Before pneumoperitoneum is ascribed to bowel perforation, a thoracic source, such as pneumothorax or mechanical ventilation, or an iatrogenic source such as a recent paracentesis or a recent surgical procedure should also be considered.
Peritoneal Carcinomatosis
Diffuse metastatic seeding of the peritoneal cavity occurs commonly with abdominopelvic tumors. The most common tumors to spread by this method are ovarian carcinoma in females and stomach, pancreas, and colon carcinoma in both sexes. The preferential sites for tumor implantation are the pouch of Douglas, the right paracolic gutter, and the greater omentum. CT findings with peritoneal tumor seeding include:
- •
Ascites is usually present and is commonly loculated.
- •
Tumor nodules appear as soft-tissue masses on, or thickening of, the parietal peritoneum ( Fig. 9.4 ). Implants and involved peritoneum may show contrast enhancement.

FIG. 9.4
Peritoneal metastases.
Metastases (arrowhead s) from ovarian carcinoma to the peritoneum appear as focal areas of peritoneal thickening and nodules.
- •
The term omental cake describes the thickened nodular appearance of tumor involving the greater omentum. The tumor cake displaces bowel away from the anterior abdominal wall ( Fig. 9.5 ).

FIG. 9.5
Omental cake.
Ascites (a) is present throughout the peritoneal cavity. The parietal peritoneum (red arrows) is thickened and is enhanced following intravenous contrast medium administration, indicating that the ascites is neoplastic or inflammatory. Omental cake (yellow arrowheads ) manifests itself as a layer of irregular soft tissue that displaces bowel away from the anterior abdominal wall.
- •
Tumor nodules and enlarged lymph nodes may be seen in the mesentery ( Fig. 9.6 ).

FIG. 9.6
Mesenteric carcinomatosis.
Tumor nodules (T) from intraperitoneal spread of ovarian cancer cause diffuse thickening of the folds of the small bowel mesentery.
- •
Thickening and nodularity of the bowel wall is due to tumor implantations on the bowel serosa.
- •
Minute implants, which may be painfully obvious and diffuse at surgery, are commonly missed by CT owing to their small size. The presence of ascites in patients with known abdominopelvic tumor, especially ovarian carcinoma, should be regarded as suspicious for peritoneal seeding, even if distinct tumor nodules are not evident. Calcification of tumor implants may aid in their CT identification. Calcified peritoneal carcinomatosis is most commonly associated with serous adenocarcinoma of the ovary, colon, or stomach.
- •
Tuberculous peritonitis mimics peritoneal carcinomatosis and may cause calcification of the peritoneum. Thickening of the peritoneum caused by tuberculous peritonitis is diffusely smooth, whereas that of peritoneal carcinomatosis is irregular and nodular.
Primary Neoplastic Diseases of the Peritoneum and Mesentery
Primary neoplastic diseases of the peritoneum are rare and diverse, with nonspecific imaging characteristics that mimic other lesions, particularly carcinomatosis:
- •
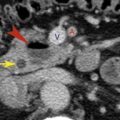
Although most malignant mesotheliomas originate in the pleura, 20% to 40% of mesotheliomas arise within the abdomen. Mesothelioma is a rare tumor with a rapidly fatal course. Asbestos exposure is a significant risk factor. CT shows an enhanced solid tumor in the mesentery, in omentum, or on peritoneal surfaces ( Fig. 9.7 ). It may cause diffuse irregular thickening of the peritoneum, multiple small nodules, or a focal peritoneal mass. Ascites is present in most cases.

FIG. 9.7
Peritoneal mesothelioma.
Tumor nodules (red arrowheads) on peritoneal surfaces are apparent. The appearance is indistinguishable from peritoneal carcinomatosis, but biopsy confirmed peritoneal mesothelioma. Adenopathy (yellow arrow) is seen adjacent to the esophagus.
- •
Multilocular mesothelioma appears as a large (>10 cm) multicystic mass with enhancing septa. The lesion mimics metastatic cystadenoma of the ovary. The clinical course is benign or indolent.
- •
Desmoplastic small round cell tumor is an aggressive malignancy that mimics Wilms tumor. It occurs most commonly in patients aged 15 to 25 years. CT shows diffuse intra-abdominal soft-tissue masses with peritoneal thickening.
- •
Primary serous carcinoma of the peritoneum is an extraovarian malignancy that occurs in postmenopausal women. CT shows diffuse peritoneal tumor and marked ascites with absence of an ovarian mass. Calcification of the tumor nodules is common (30%).
- •
Lymphoma may be primary to the peritoneum or secondary to widespread disease. Primary peritoneal lymphoma occurs nearly always in immune compromised patients. CT ( Fig. 9.8 ) reveals diffuse thickening of the peritoneum, multiple peritoneal nodules, ascites, and often extensive adenopathy.

FIG. 9.8
Peritoneal lymphoma.
Postcontrast CT revels homogeneous soft-tissue thickening of peritoneal surfaces (arrowheads) and soft-tissue mass infiltrating the perigastric and perisplenic areas (fat arrow) . A focus of lymphoma is also present in the liver (skinny arrow) .
Abscess
CT is commonly performed to search for and plan percutaneous or surgical drainage of suspected abdominal and pelvic abscesses. Once an abdominal or pelvic abscess has been found, percutaneous aspiration confirms the diagnosis and provides material for culture. Image-directed catheter placement is commonly used for drainage (“pus busting”). Most abscesses occur as complications of abdominal trauma, surgery, pancreatitis, or bowel perforation (ruptured appendicitis, diverticulitis). Intraperitoneal abscesses are commonly located in the pelvic cavity and the subphrenic and subhepatic spaces. CT features of abscess include:
- •
Most abscesses appear as loculated fluid collections displacing bowel and adjacent organs. Internal debris, fluid-fluid levels, septations, sometimes air-fluid levels, or bubbles of air ( Fig. 9.9 ) are often present within the collection.

FIG. 9.9
Subphrenic abscess.
A postoperation abscess (Ab) is seen as a fluid collection between the diaphragm and the liver. Mass impression on the liver is evidence of fluid loculation. An air-fluid level (arrow) is evidently caused by gas-producing Escherichia coli . This abscess was successfully treated by CT-guided percutaneous catheter drainage.
- •
Definable walls with irregular thickening are usually identifiable.
- •
Nearby fascia is thickened, and fat planes are infiltrated or obliterated because of inflammation.
- •
Ascites, pleural effusions, and lower lobe pulmonary infiltrates commonly accompany abdominal abscesses.
- •
Any fluid collection within the abdomen is suspect in patients in whom infection or abscess is suggested clinically. Fine-needle aspiration is a safe and definitive way to exclude or confirm the diagnosis.
Cystic Abdominal and Pelvic Masses
Cystic masses in the abdomen and pelvis commonly present challenges in diagnosis. Differential considerations include:
- •
Abscess—evidence of loculation, air bubbles, air-fluid levels.
- •
Loculated ascites—displaces organs and tissues.
- •
Pancreatic pseudocyst (see Chapter 13 ).
- •
Ovarian/paraovarian cyst/cystic tumor (see Chapter 18 ).
- •
Lymphocele—a cystic mass containing lymphatic fluid that occurs as a complication of surgery or trauma that disrupts lymphatic channels. It may be of any size and appears days to years after surgery.
- •
Cystic lymphangioma—a congenital counterpart of lymphocele believed to arise from congenital obstruction of lymphatic channels ( Fig. 9.10 ). Most are thin walled and may be unilocular or multilocular. Attenuation ranges from water to fat density. Mesenteric cysts are cystic lymphangiomas of the mesentery. Omental cysts are less common cystic lymphangiomas of the greater omentum.

FIG. 9.10
Cystic lymphangioma.
Coronal CT image showing a well-defined cystic mass (C) that is centered in the mesentery and displaces loops of small bowel without causing bowel obstruction. Pathology following surgical removal revealed a mesenteric cystic lymphangioma. Note the uniform low attenuation and imperceptibly thin wall.
- •
Enteric duplication cysts are a congenital focal cystic malformation of the gastrointestinal tract lined with gastrointestinal mucosa. They are usually attached to normal bowel and occur along the mesenteric border. On CT they appear as round or oval fluid-filled masses with a mildly enhanced wall. Often asymptomatic, reported complications include bowel obstruction, volvulus, perforation, and intussusception.
- •
Cystic teratoma may arise in the retroperitoneum, mesentery, or omentum. CT shows a complex cystic and solid mass with areas of water and fat attenuation and calcifications.
- •
Peritoneal inclusion cyst (see Chapter 18 ).
- •
Spinal meningeal cyst (see Chapter 18 ).
Vessels
Anatomy
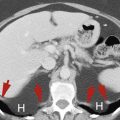
The abdominal aorta descends anterior to the left side of the spine to its bifurcation at the level of the iliac crest. The normal aorta does not exceed 3 cm in diameter and tapers progressively as it proceeds to its bifurcation. The inferior vena cava (IVC) lies to the right of the aorta. On axial imaging its shape ranges from round to oval to slit-like depending on the breath-holding technique and intravascular fluid balance. The common iliac arteries and veins appear oval in cross section as they diverge from the midline. The common iliac vessels bifurcate at the pelvic brim, which one identifies by noting the shape of the sacrum change from convex anteriorly (the sacral promontory) to concave. The external iliac vessels course anteriorly to the inguinal triangle, whereas the internal iliac (hypogastric) vessels have many small branches in the posterior pelvis. The iliac arteries normally do not exceed 1.5 cm in diameter.
The celiac axis originates from the anterior aspect of the aorta at the level of the aortic hiatus in the diaphragm. The superior mesenteric artery originates anteriorly from the aorta 1 cm below the celiac axis. The renal arteries arise from the lateral aspect of the aorta within 1 cm of the superior mesenteric artery. The inferior mesenteric artery is a tiny anterior branch off the aorta just above the bifurcation.
Anatomic Variations
A number of vascular anomalies must be recognized to avoid misinterpretation as abnormalities:
- •
Duplication of the IVC ( Fig. 9.11 ) may be identified as a persistent left IVC extending between the left common iliac vein and the left renal vein on the left side of the aorta. The enlarged left renal vein drains into the normal suprarenal IVC on the right. The right common iliac vein continues as the right IVC until it is joined by the left renal vein carrying blood from the left leg.

FIG. 9.11
Duplication of the inferior vena cava.
Sequential CT images show the persistent left inferior vena cava (IVC) (red arrowheads) extends as the continuation of the left common iliac vein (fat red arrow) along the left side of the aorta (A) to end in the left renal vein (curved arrow) , which drains into the normal right suprarenal IVC (skinny black arrow) . The normal right IVC (black arrowheads) extends from the right common iliac vein (fat black arrow) to follow its normal course through the liver. Blood flow from the left IVC flows into the right IVC via the left renal vein.
- •
The left IVC results from persistence of the left supracardinal vein with regression of the right supracardinal vein. The left IVC is formed by confluence of the common iliac veins, ascends to the left of the aorta, and joins the left renal vein, which drains into the normal suprarenal IVC on the right.
- •
Left renal veins may course posterior instead of anterior to the aorta ( retroaortic left renal vein ) ( Fig. 9.12 ), or duplicated left renal veins may course both anterior and posterior to the aorta (circumaortic left renal veins).