Endometrioma . (a) Transvaginal ultrasound demonstrates the characteristic diffuse, low-level echoes of an endometrioma. (b) Axial T1-weighted fat-saturated MRI demonstrates high signal intensity characteristic of an endometrioma. Fat saturation is important to confirm that T1 shortening is not due to the presence of fat. (c) Axial T2-weighted MRI image obtained at the same level shows diffuse hypointensity (i.e., T2 shading) of the endometrioma
Fine Needle Aspiration (FNA)
Sheets and clusters of cuboidal endometrial glandular cells (Fig. 6.2).
Clusters of spindled endometrial stromal cells.
Hemosiderin-laden macrophages.
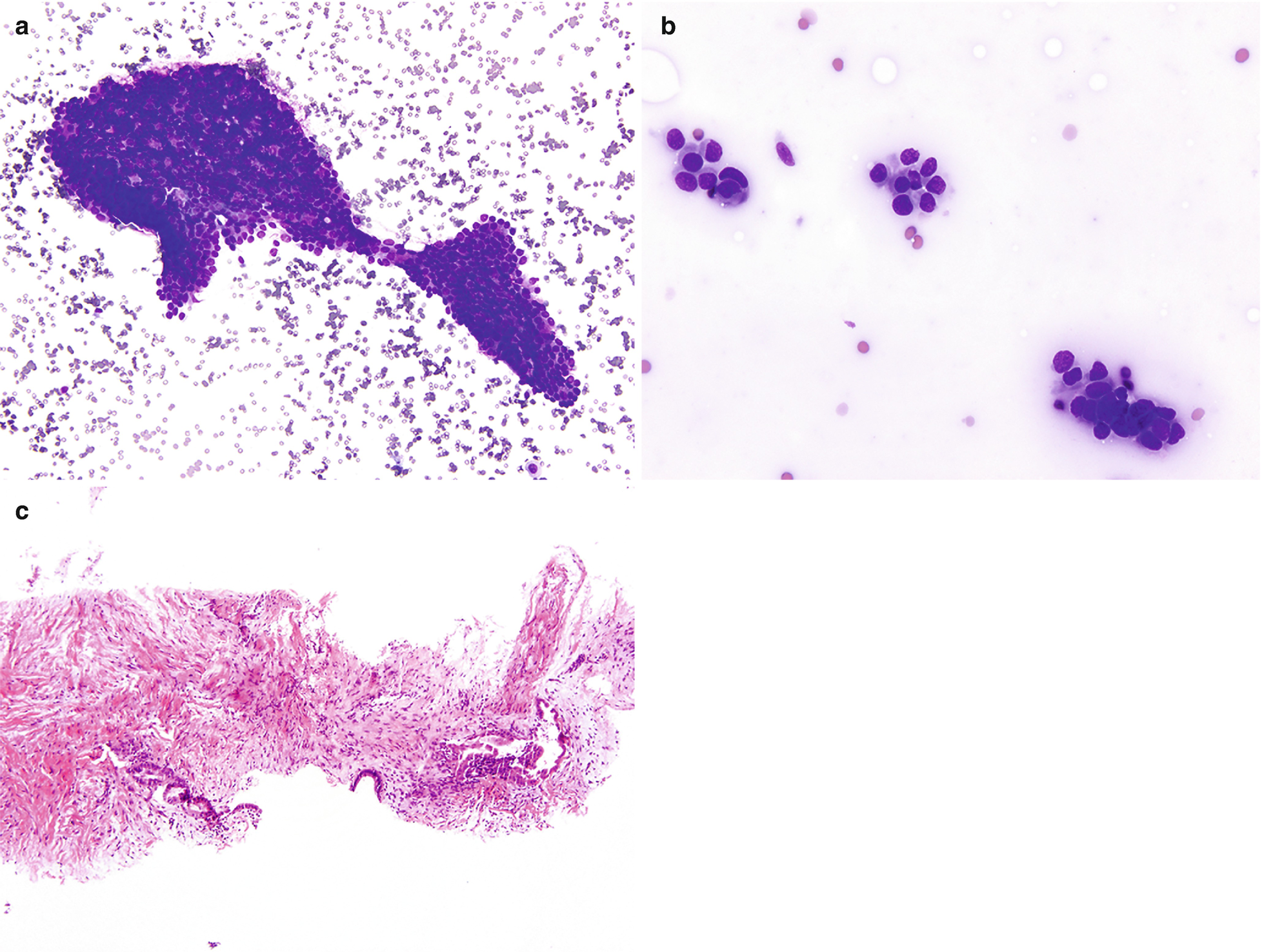
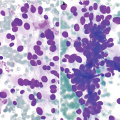
Endometriosis . (a) Clusters of benign cuboidal endometrial cells as seen in this example of endometriosis involving the peritoneum in a 27-year-old patient with a history of vague abdominal pain (DiffQuik stain, 200×). (b) Occasionally atypia can be seen in the glandular cells, as seen in this case, and care must be taken not to misinterpret this as malignancy (DiffQuik stain, 400×). (c) This histological section from a core biopsy of an abdominal nodule in a 34-year-old woman shows simple endometrial glands composed of bland cuboidal cells and the subepithelial loose endometrial type stroma involving fibrous tissue (hematoxylin and eosin [H&E] stain, 100×)
Core Biopsy
Simple endometrial glands composed of bland cuboidal cells within endometrial type stroma made up of bland spindle cells within a loose matrix (Fig. 6.2c).
Endosalpingiosis
Clinical
Usually an incidental finding, found at the time of surgery for other indications.
Similar to endometriosis; also usually involves the pelvic region (ovarian surface, uterine serosa, peritoneal surface, and omentum), but in rare instances it can be found elsewhere.
Radiology
Ultrasound
Computed Tomography
CT demonstrates masses in the abdomen and/or pelvis (Fig. 6.4), which are cystic. They may grow in size with time.
Magnetic Resonance Imaging
The MRI findings are similar to those from CT. T1-weighted fat-suppressed postcontrast images are particularly helpful to evaluate for peripheral enhancement and no central enhancement. These are T2 hyperintense. They may have internal septations [6].
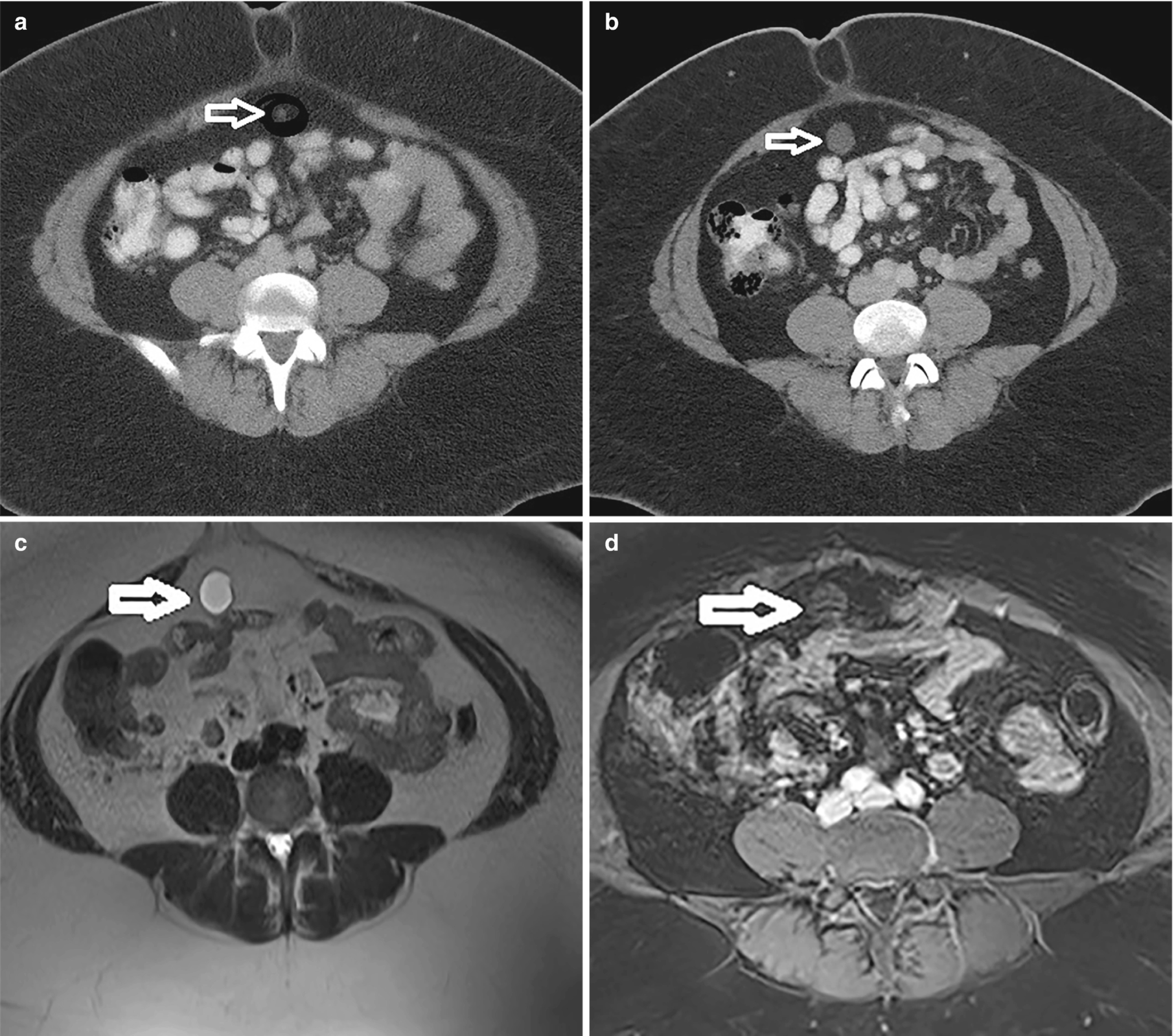
Endosalpingiosis (white arrow). (a) CT demonstrating a very small fluid attenuating structure at baseline. (b) CT 2 years later demonstrates a larger rounded structure deep to the umbilicus. (c) T2-weighted MRI demonstrates a T2 hyperintense rounded structure. (d) Post-contrast T1-weighted MRI demonstrating T1 hypointense structure with minimal rim enhancement
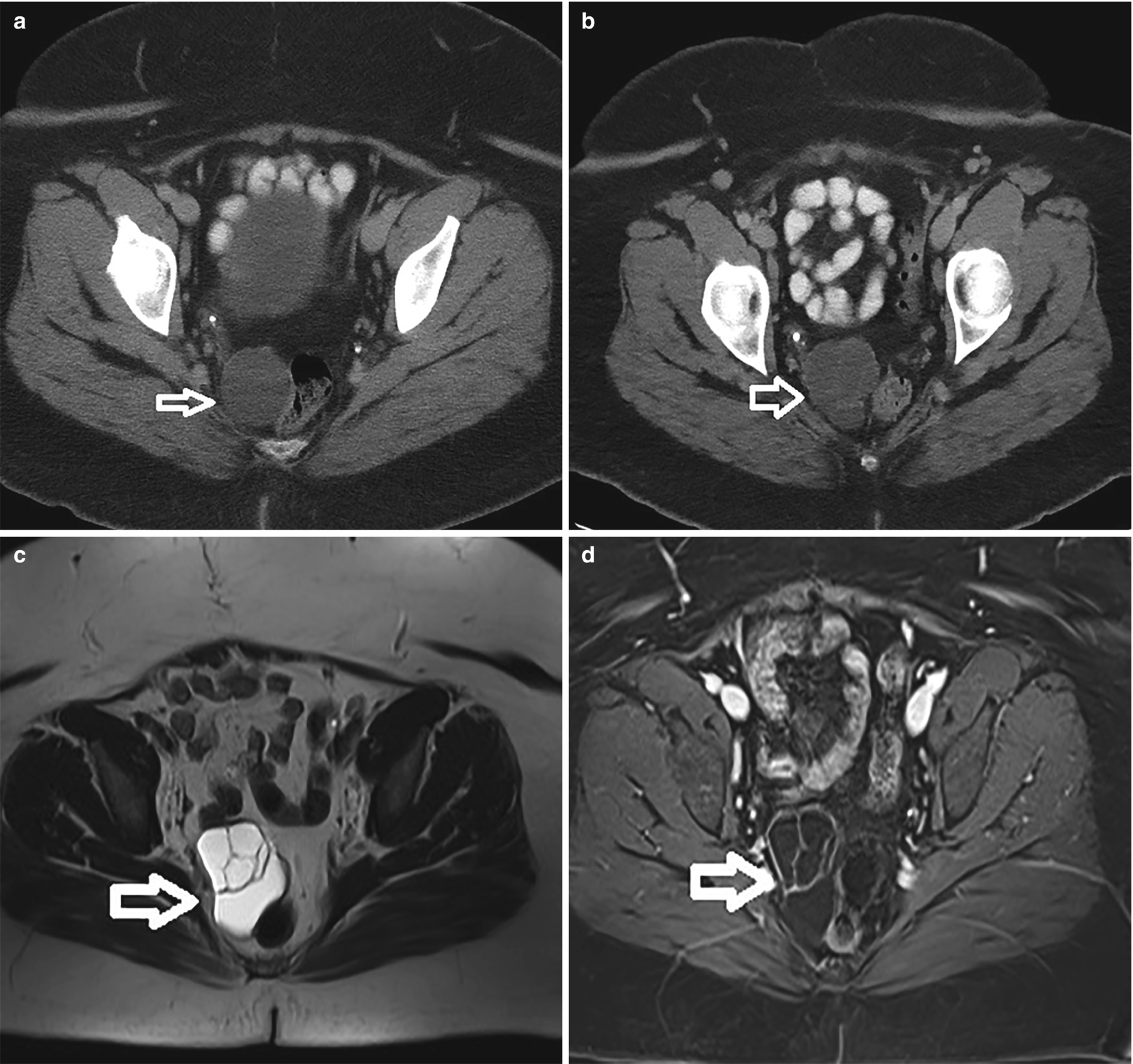
Endosalpingiosis (white arrow). (a) CT demonstrating a small fluid attenuating structure at baseline. (b) CT 2 years later demonstrates a larger multilobulated structure deep in the posterior right pelvis. (c) T2-weighted MRI demonstrates a T2 hyperintense complex cystic structure. (d) Post-contrast T1-weighted MRI demonstrating T1 hypointense structure with minimal rim and internal septal enhancement
Image-Guided Biopsy
Since this method shows no definite differentiating features from other cystic peritoneal/pelvic masses, US or CT-guided biopsy might be necessary.
Fine Needle Aspiration
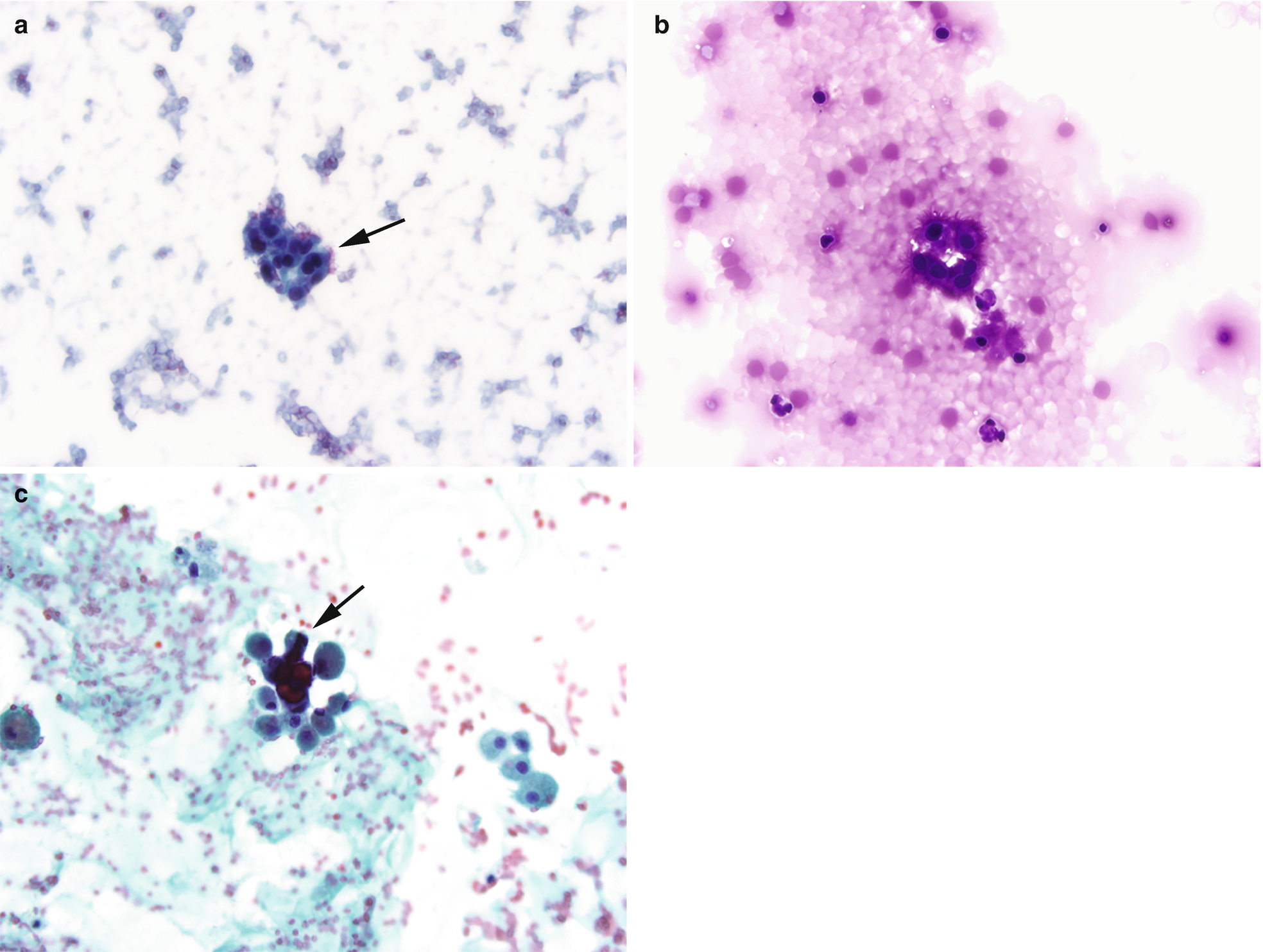
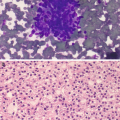
Endosalpingiosis is similar to endometriosis but instead of the presence of ectopic endometrial tissue, it is characterized by the presence of benign glands and cysts lined by ciliated, fallopian tube-like epithelium. (a) Cytomorphologically, it is similar to endometriosis with clusters of bland cuboidal cells, however the presence of cilia (arrows) is a distinctive feature (Pap stain, 400×). (b) Endosalpingiosis. Another distinction from endometriosis is that endosalpingiosis is generally asymptomatic and found incidentally during surgery or imaging for other indications. In this 45-year-old woman with a history of diverticulitis, a 2.5 cm nodule was found in the mesentery. Fine needle aspiration revealed clusters of bland ciliated glandular cells, compatible with endosalpingiosis (DiffQuik stain, 400×). (c) In some cases, psammoma bodies, defined as calcifications with lamellated concentric bands generally found in conditions with papillary architecture, may also be found, as seen in this example (arrow) (Pap stain, 400×)
Benign Ovarian Cysts
Clinical
Can be asymptomatic and found incidentally (e.g., during infertility work-up, pregnancy).
Larger cysts may present with nonspecific symptoms such as dull or sharp pelvic pain, abdominal fullness, or heaviness and bloating.
Radiology
Ultrasound
On US, cysts are thin-walled well-circumscribed lesions that are anechoic with posterior acoustic enhancement.
Computed Tomography
On CT, cysts do not demonstrate enhancement and are water attenuated.
Magnetic Resonance Imaging
On MRI, cysts contain simple fluid that is low signal on T1-weighted images and high signal on T2-weighted images. They contain a uniform thin, dark wall on T2. Again, there is no internal enhancement, although enhancement of the thin wall is typical [8].
Fine Needle Aspiration
The main cytological distinction to be made is between functional and nonfunctional epithelial-lined cysts. The former includes follicular and corpus luteum cysts, while the latter encompasses endometriotic cysts, cystic surface epithelial tumors of the ovary, and mature cystic teratomas.
Functional cysts: characterized by granulosa cells which are small, with scant indistinct cytoplasm, round to oval nuclei and coarsely granular chromatin. When degenerated, granulosa cells may mimic macrophages due to the presence of cytoplasmic microvesicles. Luteinized granulosa cells are larger and have ill-defined cell borders with abundant foamy or granular eosinophilic cytoplasm and eccentric nuclei with fine chromatin and small nucleoli. In corpus luteum cysts, luteinized cells may be seen in association with fibrin, degenerate erythrocytes], fresh blood, and hemosiderin-laden macrophages. Granulosa cells are positive for inhibin.
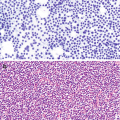
Follicular cysts are variably cellular, clusters of bland follicular cells with foamy to granular cytoplasm, small round nuclei with condensed chromatin as well as larger round nuclei with vesicular chromatin with more finely dispersed chromatin, and macrophages (Fig. 6.6) [7, 9]. They may pose a potential diagnostic pitfall due to high cellularity, cells with hyperchromatic nuclei containing conspicuous nucleoli, and the presence of mitotic figures, which may be numerous.
Nonfunctional cysts: endometriotic cysts, cystic surface epithelial tumors of the ovary, and mature cystic teratomas They often have scant cellularity, few bland cuboidal ovarian surface/mesothelial-like cells, and macrophages.
Endometriotic cysts yield brownish/chocolate-colored fluid containing hemosiderin-laden macrophages with endometrial stromal and/or epithelial cells present in some cases. Often a specific interpretation beyond hemorrhagic cyst with a differential diagnosis will be rendered if all components are not seen.
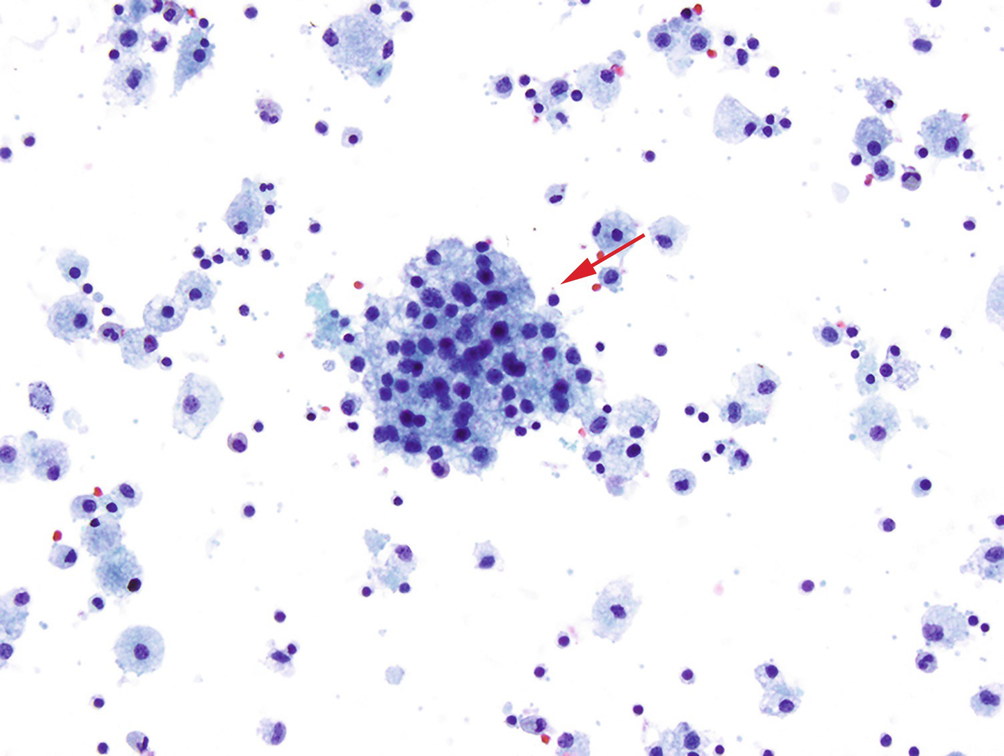
Ovarian cysts can arise in a variety of benign and neoplastic conditions but the benign variety fall into two general categories: nonfunctional inclusion cysts, which arise from ovarian surface epithelium, and/or fallopian tube epithelium and functional cysts, including follicular and corpus luteum cysts. Cyst fluid aspirate shows a background of foamy macrophages and a cluster of follicular cells with bland round nuclei and vacuolated cytoplasm (arrow) characteristic of a follicular cyst (Pap stain, 200×)
Malignant Conditions
Malignant Mesothelioma
Clinical
Strong association with previous asbestos and other occupational exposure (shipyards, military facilities, and industrial processing job sites). Occurs most commonly in the pleura and to a lesser degree in the peritoneum.
Early in the disease it is generally asymptomatic, but with progression and late stage disease, patients can present with abdominal pain, ascites, and weight loss.
Radiology
Ultrasound/Biopsy
Evaluating for peritoneal mesothelioma with US is difficult except in the setting of large-volume ascites. Similar to the case in omental metastases, the peritoneal fluid provides a good acoustic window to evaluate for nodularity [10]. Centrally located tumors are poorly imaged with US because of the acoustic impedance of bowel gas and mesenteric fat. Figure 6.7 demonstrates an US of the left upper quadrant with echogenic soft-tissue thickening. US is also the most commonly used method for imaging guidance to perform biopsies of these masses.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree