Fig. 32.1
(a–c) Anatomy of greater trochanter with tendinous insertion sites and bursae. (a) The three main bursae and their positions. (b) Geometry of greater trochanter with different facets. (c) Footprints of gluteus medius and minimus tendon insertions (a–c: Used with permission from Domb BG, Nasser RM, Botser IB. Partial-thickness tears of the gluteus medius: rationale and technique for trans-tendinous endoscopic repair. Arthroscopy. 2010; 26(12):1697–1705)
The most superficial structure of the peritrochanteric space is a fibromuscular sheath composed of the gluteus maximus, tensor fascia lata, and ITB. The gluteus maximus inserts into the posterior aspect, while the tensor fascia lata inserts into the superior and anterior aspects of the ITB. The fascia lata that encloses these structures extends superiorly without muscle attachment to the tubercle of the iliac crest. Just distal to the hip joint, the ITB has a thick expansion—the gluteus maximus sling—that inserts on the posterolateral femur. The ITB crosses the knee joint distally and inserts onto Gerdy’s tubercle on the anterolateral aspect of the proximal tibia.
The hip abductors consisting of the gluteus medius and minimus have been referred to as the “rotator cuff tears of the hip” [14, 19]. Table 32.1 shows similarities and differences between shoulder and hip rotator cuffs. The smaller gluteus minimus originates from the anterior inferior iliac spine (AIIS) to the posterior inferior iliac spine (PIIS), runs parallel to the femoral neck, and inserts into both the hip capsule and lateral facet beneath the gluteus medius [20]. The fan-shaped gluteus medius originates from the anterior superior iliac spine (ASIS), outer edge of the iliac crest, and back to the posterior superior iliac spine (PSIS). Depending on the source, it has two or three insertion points on the greater trochanter: the superoposterior facet has a thick insertion from the central posterior portion of the muscle, a thin, broad lateral component inserts onto the lateral facet, and a continuation onto the anterior facet that is not visible macroscopically [16, 21, 22].
Table 32.1
Similarities and differences between shoulder and hip rotator cuffs
Shoulder rotator cuff | Hip rotator cuff | |
|---|---|---|
Functional anatomy | ||
Internal rotator | Subscapularis | Iliopsoas |
Stabilizers and rotators, initiation and assistance in abduction | Supraspinatus and infraspinatus | Gluteus medius and minimus |
Abduction | Deltoid | Tensor fascia lata |
Clinical presentation | Pain with motion | Tenderness over lateral aspect of hip |
Tenderness | Weakness in abduction | |
Weakness in abduction | ||
MRI/ultrasound | Visualized on MRI and ultrasound | Visualized on MRI and ultrasound |
Mechanism | Degenerative tearing | Degenerative tearing |
Acute trauma | Acute trauma | |
Arthroscopic evaluation | Articular tears can be visualized as either exposed footprint or delamination | Undersurface tears cannot be easily visualized |
Using electromyography (EMG), Gottschalk et al. describe the primary function of the gluteus minimus and posterior gluteus medius as a stabilizer of the femoral head in the acetabulum during motion and gait [23]. The anterior and middle portions of the gluteus medius have a vertical pull and help initiate abduction, whereas the tensor fascia lata is the major abductor of the hip.
Differential Diagnosis
The diagnosis of GTPS can be complicated due to the multiple possible sources of pain surrounding the hip girdle. The differential diagnosis includes intra-articular hip pathology, extra-articular hip pathology, and sources outside of the hip. Intra-articular sources include labral tears, loose bodies, femoroacetabular impingement, capsular laxity, ligamentum teres rupture, and chondral damage. Extra-articular sources include stress fractures, piriformis syndrome, and neoplasm [24]. Sources of hip pain that are outside the hip include pathology of the superior gluteal nerve, meralgia paresthetica, lumbar spondylosis, and lumbar radiculopathy [20]. In regard to the latter two, a limp and hip abductor weakness may be present along with radiating pain, similar to GTPS. Also, patients with a history of total hip arthroplasty, especially through an anterolateral approach, may have iatrogenic injury to the abductor mechanism or its innervations [20]. A detailed history, physical exam, and the appropriate imaging will help to narrow the differential.
Imaging
Imaging has long been thought to be largely unnecessary for the diagnosis of GTPS. However, with cases refractory to standard conservative management, imaging can be very helpful. The most common imaging modalities include plain radiographs, ultrasound, and MRI.
Radiographs do not typically show specific abnormalities with regard to GTPS. A trochanter further lateral than the lateral border of the iliac crest has been shown to be a predisposing risk factor for GTPS [11]. Intrabursal calcifications, abductor calcific tendinosis, or enthesophytes of the greater trochanter may be seen but are not specific. Radiographs are also useful to rule out fracture or osteoarthritis of the hip.
Ultrasound can also aid in the diagnosis of GTPS. It can be especially useful in diagnosing abductor tendon pathology, as it has been shown to have a sensitivity of 79 % and positive predictive value of 100 %, rivaling that of MRI [25]. Dynamic ultrasound can be used to visually confirm the diagnosis of external snapping hip [26].
MRI is currently considered the gold standard for diagnosing GTPS [9, 27]. In the setting of GTPS, patients with abnormalities seen in T2-weighted images are significantly more likely to have abductor tendinopathy [28]. Kingzett-Taylor et al. reviewed 250 hip MRIs for pain involving the buttock, lateral hip, and groin [29]. Gluteus medius and minimus tears were seen in 35 studies. They concluded that tendinopathy is a frequent cause of GTPS and likely associated with trochanteric bursitis. However, another study cautioned that MRI might have a false-positive rate as high as 88 % when evaluating abductor tendon tears [30].
Treatment
Conservative management is effective for the great majority of greater trochanteric pain syndrome cases. Treatment begins with rest, refraining from pain exacerbating activities, ice, nonsteroidal anti-inflammatory medications (NSAIDs), and physical therapy. Therapy focuses on stretching and strengthening of the iliotibial band (ITB) and gluteal muscles. Independent or a combination of these measures can have a cure rate of greater than 90 % [31]. For refractory cases, glucocorticoid injections have been shown to return patients to their baseline activity level 49–100 % of the time [32]. Low-energy shock-wave therapy has also been shown to have superior improvement in visual analog scale and Harris hip scores compared with the primary outcome of other conservative measures [33, 34]. All of these options should be exhausted before surgical options are considered.
As with many surgical procedures, open techniques have given way to arthroscopic and endoscopic solutions. Hip arthroscopy has made significant advances since its introduction in 1931 and popularization during the late 1980s and early 1990s [35–37]. These surgical techniques and other technological achievements have helped expand hip arthroscopy to extra-articular anatomic regions, which is considered peritrochanteric endoscopy. The peritrochanteric endoscopic borders are the tensor fascia lata and ITB laterally, the abductor tendons superomedially, the vastus lateralis inferomedially, and the gluteus maximus muscle superiorly and its tendon posteriorly [8].
Hip arthroscopy and peritrochanteric endoscopy can be utilized based on surgical goals; however, portal placement, visualization pearls, and other procedural nuances have been described. Voos et al. suggest using the same portals used for evaluation of central and peripheral compartment disorders with the anterior portal offering best access to the peritrochanteric space [38]. This portal is made 1 cm lateral to the anterior superior iliac spine within the interval of the tensor fascia lata and sartorius. For optimal access, safety, and hemostasis, balloon dissection has been shown to be superior to blunt dissection [39]. A standard 30° or 70° arthroscope is sufficient for peritrochanteric endoscopy.
Case-based examples of common causes of greater trochanteric pain syndrome will be outlined in this chapter. Multiple figures will be utilized to reveal the relationship between MRI imaging and intraoperative arthroscopy for each case. The three cases include:
1.
Recalcitrant trochanteric bursitis
2.
External snapping hip
3.
Gluteus medius tear
Case 1: Recalcitrant Trochanteric Bursitis
History/Exam
A 45-year-old female presented to orthopedic clinic with an 18-month history of right hip pain. The patient stated that the pain began while running. Initially, running was the only activity that bothered her; however, she began to experience pain with prolonged standing, sitting with the affected leg crossed, or lying on the affected side. She had an active lifestyle that included running 2 miles 6 days per week but has since stopped. She located the pain to the lateral aspect of her hip and noted that it radiated down the lateral aspect of the thigh. She quantified her pain as a 5 on a scale of 0–10. The pain was intermittent in nature.
She was initially seen by a chiropractor and was prescribed a course of physical therapy that included range of motion and strengthening exercises. This, however, exacerbated the patient’s pain to a 9 out of 10, and she discontinued physical therapy after 1 month. She subsequently sought treatment by her primary care physician who again recommended physical therapy with the addition of NSAIDs. However, due to the prior failure of physical therapy and an allergy to NSAIDs, she could not complete her recommendations.
On physical examination, her gait was normal without signs of Trendelenburg. The skin was normal—no ecchymosis, erythema, or swelling. There was point tenderness to palpation over the greater trochanter. Her hip range of motion was as follows: 120° of flexion, 10° of internal rotation with pain, 30° of external rotation, and 30° of abduction. She experienced pain with resisted abduction and internal rotation, but there were no signs of weakness. She had a positive FABER test and Ober’s test. Anterior impingement and internal snapping were also evident. She was otherwise neurovascularly intact throughout the lower extremity.
After failing physical therapy and other conservative measures, the patient was offered a corticosteroid injection with local anesthetic into her right trochanteric bursa. The injection was conducted under ultrasound guidance. Within 30 min, the patient’s pain had substantially improved. The injection provided 3–4 weeks of pain relief before symptoms began to return.
Imaging
Standard X-rays were obtained. Views including anterior/posterior pelvis, right hip false profile, bilateral Dunn views, and a right hip cross-table lateral. The joint spaces were well preserved. The patient did have a mild femoral cam lesion and a small crossover sign. There was no sign of antecedent trauma to the trochanter.
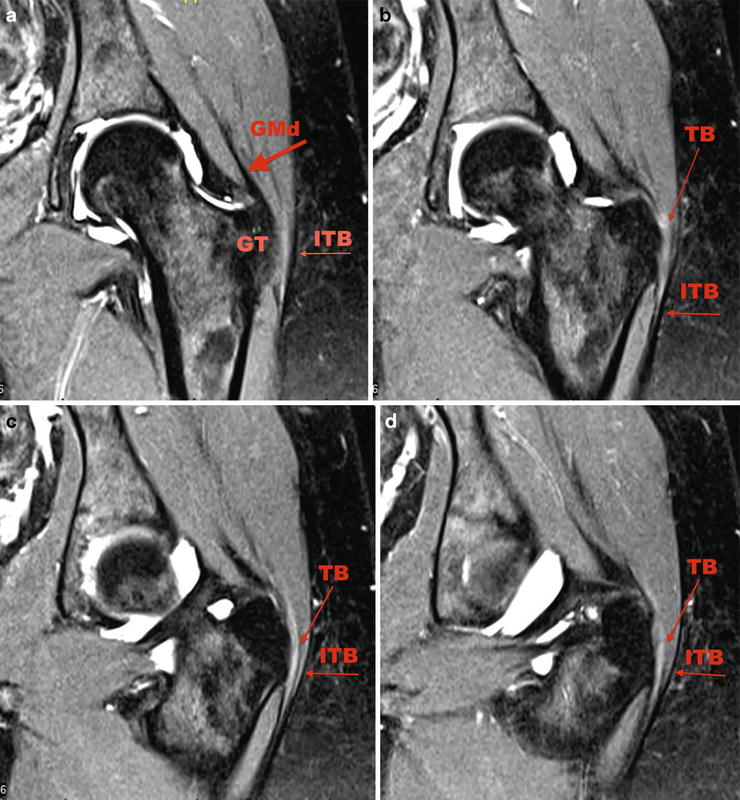
Typically, the diagnosis of trochanteric bursitis is clinical; however, due to the recalcitrant nature of the problem and signs of both intra- and extra-articular pathology, an MRI arthrogram was obtained to delineate other possible pathology. Coronal fat-saturated T2-weighted images are shown in Fig. 32.2a–d, and axial fat-saturated T2-weighted images are shown in Fig. 32.3a–d.
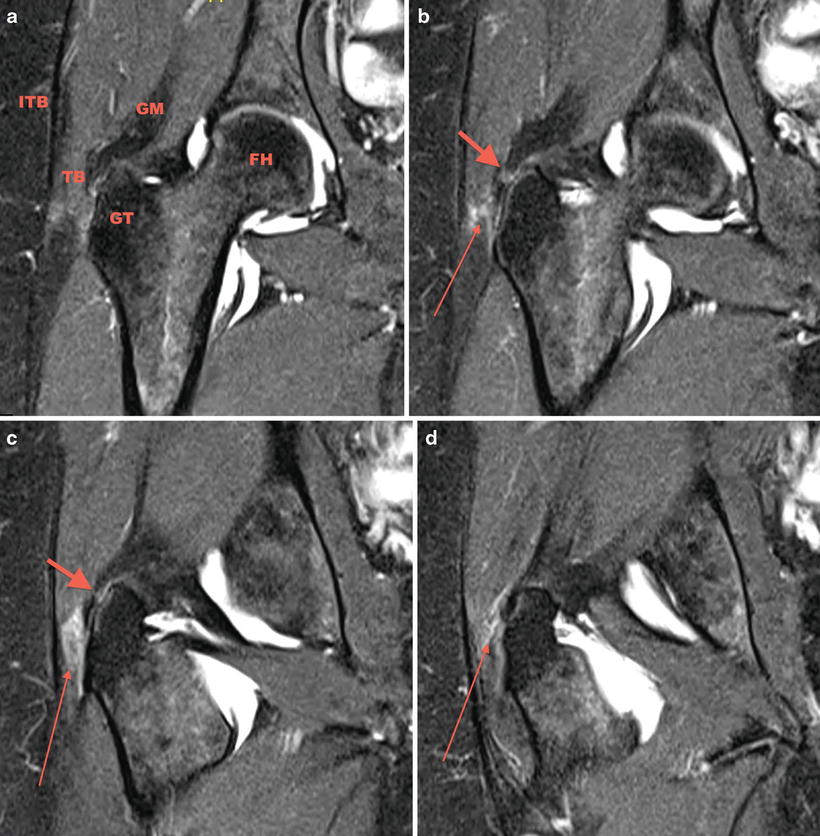
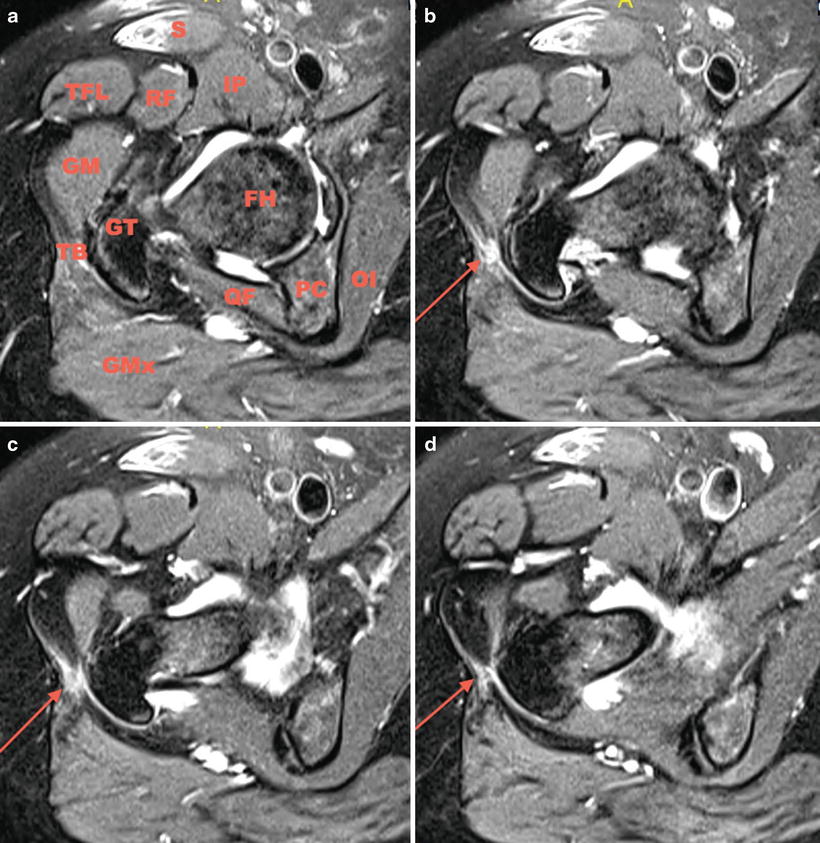

Fig. 32.2
(a) Coronal fat-saturated T2-weighted image. The anatomic structures being scrutinized: GM gluteus medius, FH femoral head, GT greater trochanter, TB trochanteric bursa, ITB iliotibial band. (b, c) Mild signal heterogeneity can be seen in the substance and at the insertion of the abductor tendons indicating tendinosis without evidence of tear evidenced by the short fat arrow. (b–d) A moderate amount of fluid and edema is seen lateral to the GT suggesting bursitis as shown by the long thin arrow

Fig. 32.3
(a) Sagittal fat-saturated T2-weighted image. Surrounding anatomic structures in clockwise direction: S sartorius, IP iliopsoas, FH femoral head, OI obturator internus, PC posterior column, QF quadratus femoris, GMx gluteus maximus, TB trochanteric bursa, GT greater trochanter, GM gluteus medius, TFL tensor fascia lata, RF rectus femoris. (b–d) A moderate amount of fluid and edema is seen lateral to the GT suggesting bursitis as shown by the long thin arrow
Figure 32.2a denotes the anatomic structures being scrutinized. A moderate amount of fluid and edema is seen lateral to the greater trochanter suggesting bursitis as shown by the long thin arrow in Figs. 32.2a–d and 32.3b–d. In addition to bursitis, mild signal heterogeneity can be seen in the substance and at the insertion of the abductor tendons indicating tendinosis without evidence of tear evidenced by the short fat arrow in Fig. 32.2b–d. A labral tear was also identified.
The patient’s clinical and radiographic presentation was consistent with trochanteric bursitis, although she also had mild signs and symptoms of femoroacetabular impingement. Given her failure to improve with conservative treatment, the patient elected to proceed with surgery and was consented for right hip arthroscopy with labral treatment, femoral osteoplasty, acetabuloplasty, and peritrochanteric endoscopy with trochanteric bursectomy and debridement. The patient was counseled that if a gluteus medius tear was identified, it would be repaired.
Arthroscopy
The patient was brought to operating room placed in the supine position on a traction table extension with a well-padded perineal post. Traction was applied to the hip under fluoroscopy. An anterolateral portal was created first, followed by a more distal lateral accessory portal. A capsulotomy was made parallel to the acetabular rim connecting the two portals. Diagnostic arthroscopy and intra-articular procedures were completed first.
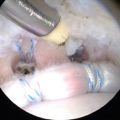
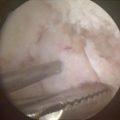
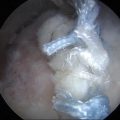
A blunt obturator was used to reinsert the arthroscope into the peritrochanteric compartment through the mid-anterior portal. A thickened band of bursa is seen being probed in Fig. 32.4. The shaver was introduced via the anterolateral portal and trochanteric bursectomy, and peritrochanteric debridement was performed. The remainder of the peritrochanteric space was examined, including the gluteus medius and maximus tendon insertions, which were found to be intact.
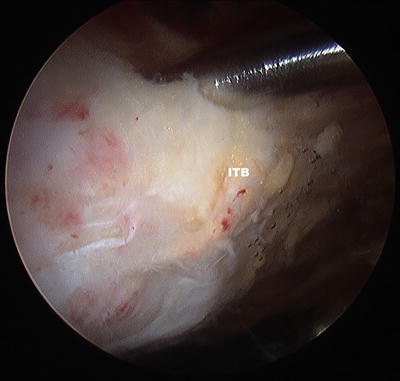

Fig. 32.4
Thickened band of bursa is seen being probed
Discussion
Trochanteric bursitis is usually self-limited and responds well to conservative treatment [2, 40]. Open or arthroscopic surgical management of this condition is effective but rarely necessary lending to a paucity of high-level research. Fox et al. retrospectively reported on 27 patients treated with arthroscopic bursectomy for recalcitrant trochanteric bursitis. At a minimum of 1 year, 23 out of 27 patients had “good or excellent” results immediately postoperative with no complications. Symptoms recurred in one patient at 1 year and two patients at 5 years.
A tight ITB rubbing over the greater trochanter is a documented etiology of trochanteric bursitis [41]. Thus, there are reports of modifying the ITB in addition to trochanteric bursectomy during arthroscopic surgery. Farr et al. performed arthroscopic bursectomy along with an ITB release in two patients. They reported that both had complete relief of symptoms and returned to their preoperative occupational and recreational activities without recurrence. Govaert et al. also advocated for release of the ITB during surgical management of GTPS [42]. They treated five patients with a follow-up of 6 weeks in which three were “satisfied” and two were “very satisfied.” One patient, however, developed a large hematoma that required open evacuation. Weinrauch et al. describe ultrasound-assisted arthroscopy to ensure adequate decompression of the peritrochanteric space [43]. Strauss et al. believe releasing the posterior one third of the ITB is only necessary when there is clinical evidence of external snapping or ITB tightness (positive Ober’s test); otherwise, bursectomy alone is sufficient [8].
Baker et al. prospectively evaluated 30 patients with recalcitrant trochanteric bursitis following arthroscopic bursectomy for a mean of 26.1 months [44]. Significant improvements in pain scores (7.2 preoperatively versus 3.1 postoperatively) and Harris hip scores (51 preoperatively versus 77 postoperatively) were noted in the 25 patients available for follow-up. Of note, the authors “often noted scuffing or irritation of the gluteus medius and minimus tendons and, occasionally frank tears of their insertions…that were treated with debridement of the edges and decompression of the area.” The precise number of patients with these findings was not mentioned; therefore, it is difficult to apply their results to recalcitrant trochanteric bursitis, specifically. Their results should more appropriately be applied to patients with the general diagnosis of GTPS. In addition, the diagnostic criteria used in this study were mainly clinical. MRIs were not routinely obtained. Of those that were available, “no attempt to correlate the MRI results with the findings at the time of surgery” was made.
An isolated diagnosis of recalcitrant trochanteric bursitis is likely rare. Bird et al. reviewed the MRIs of 24 women with GTPS finding that 62.5 % had evidence of gluteus medius tendonitis, 45.8 % with gluteus medius tears, but only 8.3 % had objective evidence of trochanteric bursitis [4]. Additionally, Long et al. retrospectively reviewed the ultrasound findings of 877 patients with GTPS [5]. Nearly 80 % (700 patients) did not have bursitis on ultrasound, while 50 % (438 patients) had abductor tendinosis, and 28.5 % (250 patients) had a thickened ITB. The case presented here had concomitant abductor tendinosis and femoroacetabular impingement with labral damage, in addition to the trochanteric bursitis. Therefore, when preoperatively planning for surgical management of recalcitrant trochanteric bursitis, there should be a high degree of suspicion for coexisting pathology in the peritrochanteric space as well as intra-articularly.
Case 2: External Snapping Hip
History/Exam
A 23-year-old female presents to the orthopedic clinic with long-standing bilateral hip pain, left side greater than the right. She states that since childhood, she has been able to “take her hip out of its socket” referring to a snapping/clunking sensation. As an adult, her pain has been increasing and is associated with same snapping during the last 4 years. Of recent, it has also begun to affect her knees. The hip pain is located on the lateral aspect of the hip, is intermittent, and is rated at 8 out of 10. She locates her knee pain to the anterolateral aspect of the knee just inferior to the joint line. Both her hip and knee pains increase with activity level. The only relieving factor she has found is ice. She has completed three courses of physical therapy of 6–8 weeks each without relief of symptoms. She has also had corticosteroid injections into bilateral greater trochanteric bursas and knee joints without improvement.
On physical examination, she appears healthy. Examination of her gait and overall alignment reveals mild genu valgum but a normal heel-to-toe gait. Her bilateral hip range of motion is as follows: 120° of flexion, 30° of internal rotation with pain, 50° of external rotation with pain, and 50° of abduction. She denies pain directly over the greater trochanters, however, does admit to tenderness over the piriformis. With the patient lying on her side, affected side up, flexing the hip while palpating the greater trochanter reveals a snapping sensation of the IT band over the trochanter. Applying a firm pressure relieves the snapping. The patient also exhibits a positive Ober’s test. Of note, the patient did also exhibit signs of internal impingement and internal snapping.
Examination of the patient’s knees does not show any effusion or erythema. Bilateral knee range of motion is 130° flexion with pain to −5° extension. She is tender to palpation over Gerdy’s tubercle of the bilateral tibias. There is no patellar instability, but there is crepitus and mild tenderness to palpation at the inferior pole of the patella. Muscle testing of the lower extremities reveals 5/5 strength bilaterally. Additionally, bilateral lower extremities are neurovascularly intact.
Imaging
X-rays included a supine AP view of the pelvis, bilateral false profile and Dunn views, and a cross-table lateral view. Joint spaces are preserved bilaterally. There is a 20 % crossover sign bilaterally.
An MRI arthrogram was conducted due to the patient’s anterior impingement symptoms in order to further delineate a labral tear—the pathology and treatment of the labral tear will not be discussed in this chapter. Figures 32.5a–d and 32.6a–d depicts coronal and axial T2 fat-saturated weighted MRI cuts moving from anterior to posterior and inferior to superior, respectively. A thin black line lateral to the greater trochanter (GT) represents the iliotibial band (ITB). The gluteus medius tendon is also identified (GMd) in Fig. 32.5a. Figure 32.5b–d depict slight thickening of the trochanteric bursa (TB) with a very mild increase in signal intensity. The iliotibial band (ITB) does not demonstrate significant abnormalities in these cuts. The axial sequences, however, are more useful to show areas of thickening of the iliotibial band in the sagittal plane. An area of thickened ITB can be seen just anterolateral to the greater trochanter in contrast to the posterolateral ITB seen in Fig. 32.6a–d.