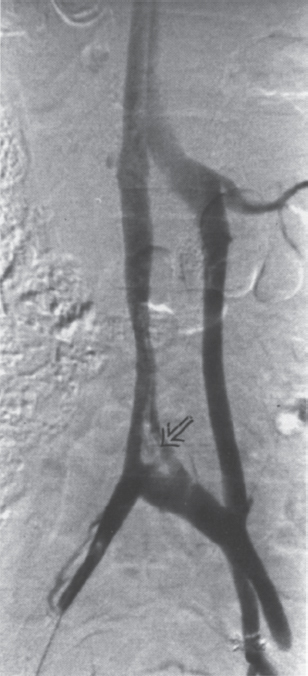
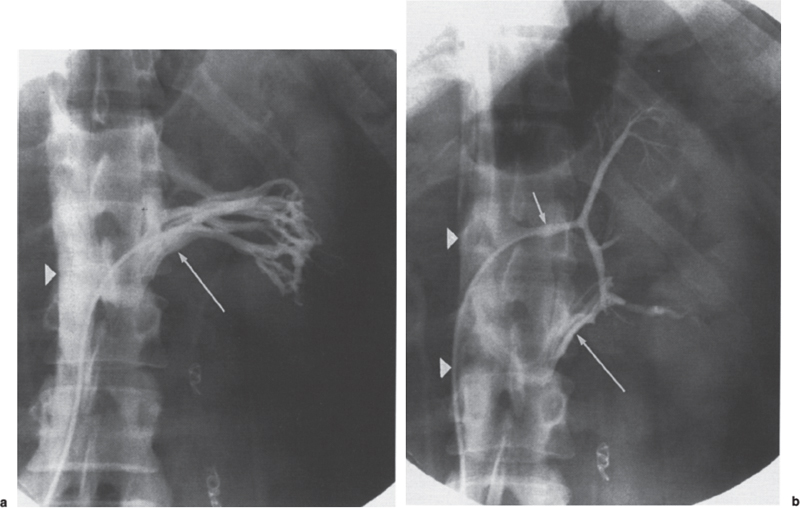
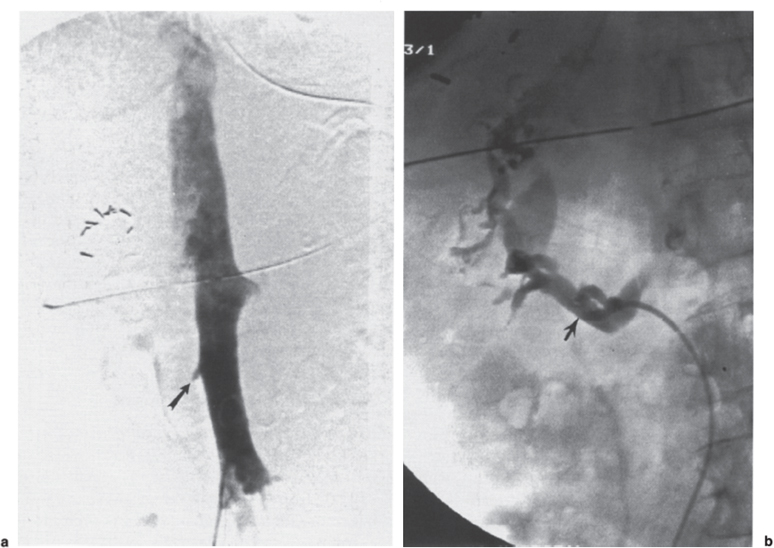
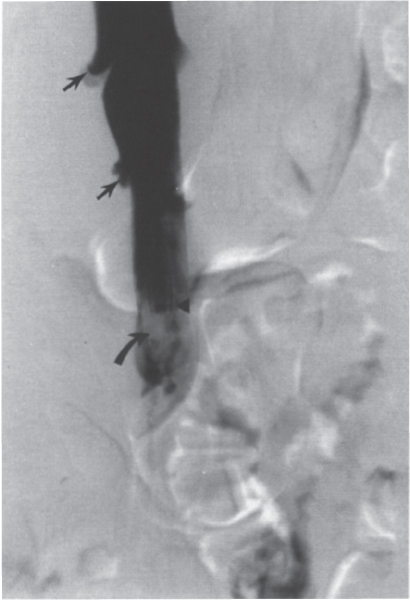
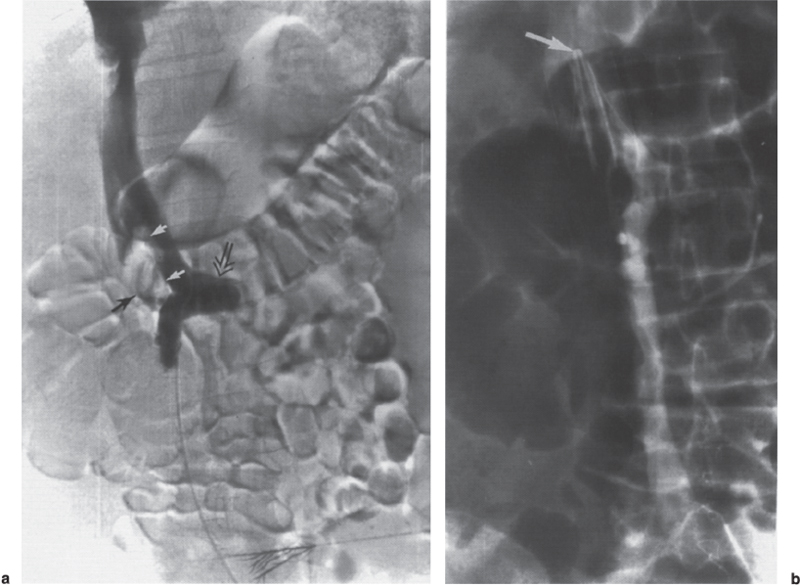
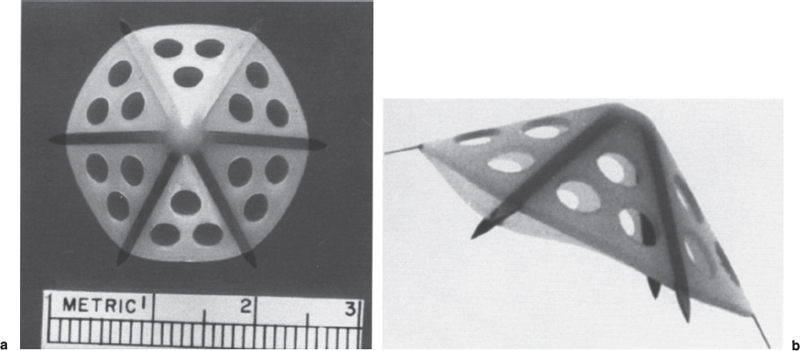
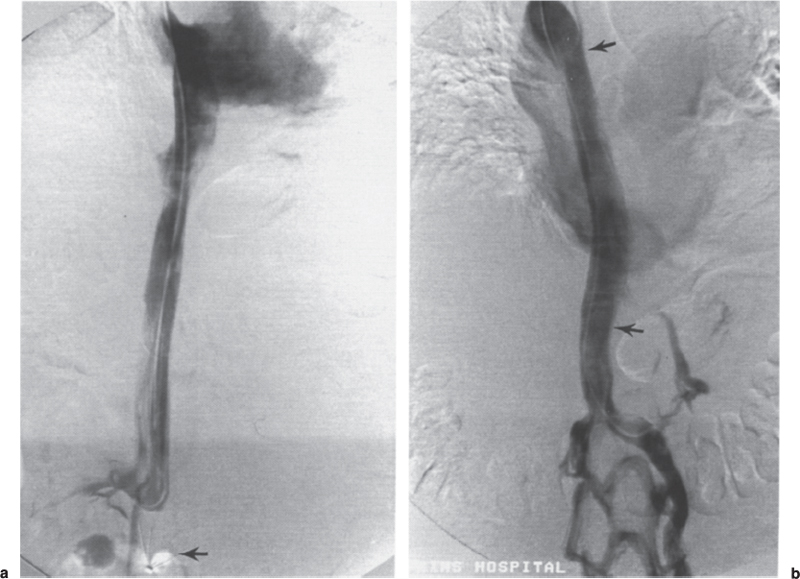
Permanent Inferior Vena Cava Filters Pulmonary embolism is a common condition with unreliable clinical indicators of occurrence, a spectrum of disease severity, and differences of opinion among clinicians as how best to diagnose this entity. The exact incidence is difficult to pinpoint. An estimated 570,000 to 630,000 cases of symptomatic pulmonary embolism occur annually, resulting in 200,000 fatalities,1,2 50,000 of which are solely attributable to pulmonary embolism.3 Although systemic anticoagulation with intravenous heparin and oral warfarin sulfate will provide adequate therapy for most patients, studies have shown that 3 to 20% of patients will experience a second pulmonary embolism while taking anticoagulants.2,4,5 In addition, the complication rate associated with anticoagulation may be as high as 26%, with a fatality rate of 5 to 12%.6–8 Many patients may have contraindications to anticoagulation therapy that include active or recent hemorrhage, peptic ulcer disease, primary or metastatic disease (particularly intracranial tumors), pregnancy, and planned or recent surgery.7–10 Although anticoagulation remains the mainstay of therapy for patients with deep venous thrombosis and pulmonary embolism, the aforementioned limitations necessitated the development of alternative therapies and lead to the development of the intraluminal inferior vena cava (IVC) filter. A common indication for placement of an IVC filter is the presence of a contraindication to anticoagulation in a patient with known pulmonary embolism or deep venous thrombosis involving the femoropopliteal veins, iliac veins, or IVC, or, less frequently, the veins of the upper extremities and intrathoracic great veins of the upper extremities and intrathoracic great veins. Contraindications to anticoagulation can occur in 25 to 77% of patients10,11 and include previous complications while taking anticoagulants, known gastric or peptic ulcer disease, guaiac positive stools, and recent surgery (particularly involving the eyes, brain, or spinal cord). Patients with severe thrombocytopenia (e.g., patients with acquired immunodeficiency syndrome, idiopathic or thrombocytopenic purpura) and hemophilia should not receive anticoagulants. Patients with an unsteady gait (e.g., Parkinson’s disease, stroke), a tendency to fall, or unreliable compliance with oral therapies also should be evaluated for potential IVC filter placement. Another indication is recurrent pulmonary embolism despite adequate anticoagulation, which event can occur in 27 to 33% of patients10,12 and is fatal in up to 9%.1,13 Complications directly attributable to anticoagulant therapy occur in about 18% of patients, necessitating its discontinuation.10,12 The insertion of an IVC filter also may be indicated for prophylaxis in high-risk patients, including those with a history of (1) deep venous thrombosis and pending lower-extremity orthopedic surgery (25% of whom may experience pulmonary embolism), (2) free-floating caval thrombus, (3) chronic pulmonary hypertension, and (4) marginal cardiopulmonary reserve.12 Prophylactic filter placement also may be indicated in the setting of trauma. Patients who have had pelvic trauma, multiple long-bone fractures, or head or spinal cord trauma are at increased risk of deep venous thrombosis and pulmonary embolism.14,15 Patients with malignancies and deep venous thrombosis or pulmonary embolism may be at a particularly high risk for complications with anticoagulant therapy. Moore et al16 reported 21 hemorrhagic events in a group of 16 such patients, 25% of which resulted in a major hemorrhage, leading to cessation of therapy or death. Recurrent pulmonary embolism occurred in 19% of the patients who received adequate anticoagulation. Prophylactic filter placement in this unique group of patients is further supported by the work of Cohen et al17 who demonstrated a 0% incidence of hemorrhagic complications and only one (2.4%) fatal pulmonary embolism in 41 patients treated in this fashion. Experiments in animal models demonstrated that septic emboli trapped in a Greenfield filter can be sterilized with antibiotic therapy.18 This technique has resulted in lower morbidity and mortality rates than when conventional therapy of caval ligation is used. Septic pulmonary emboli may originate from the lower extremities in cases of septic thrombophlebitis; from the pelvic veins following pelvic surgery (i.e., abdominal–peritoneal resection) with subsequent infection; or from the ovarian veins in cases of postpartum endometritis, obstetric and gynecologic surgery, and pelvic inflammatory disease.19 There are few, if any, absolute contraindications to placement of an IVC filter. Knowledge of the coagulation status of the patient is absolutely necessary, but with the use of the smaller filter designs (14F or less), we generally do not require full reversal of anticoagulation prior to filter placement. Orders to discontinue heparinization “on call” for the procedure are usually adequate. If the filter is placed from a transjugular approach, having the patient sit upright after the procedure while gentle pressure is applied to the access site, will decrease the time required to obtain hemostasis. Patients who are scheduled for IVC filter placement and who may require magnetic resonance (MR) imaging should have a filter placed that is nonferromagnetic. This includes the titanium Greenfield, Vena-Tech, and Nitinol IVC filters. A detailed, informed consent signed by the patient or legal guardian is always required when an IVC filter is to be placed. Coagulation studies consisting of a platelet count, activated partial thromboplastin time (aPTT), and prothrombin time (PT) are also required; but, as indicated earlier, patients on anticoagulation usually do not require full reversal of heparinization prior to the procedure. Some physicians, however, prefer that heparin (half-life, 60 to 90 minutes) be discontinued at least 2 hours before the procedure. The platelet count should be 50,000 μL or greater (normal, 150 to 400 × 103/μL). We use the anxiolytic midazolam and the narcotic fentanyl in combination for their synergistic anxiolytic and pain-relieving properties.20,21 Continuous monitoring of vital signs is absolutely mandatory when using these agents because of their respiratory depressive effect. Technique Inferior venacavography always should be performed before an IVC filter is placed. Besides providing imperative information on the size of the IVC, inferior venacavography yields additional clinically important information in up to 26% of cases.22 Following sterile preparation of the access site, sterile drapes are used to cover the patient. A standard 18-gauge single-wall entry needle (Cook, Bloomington, IN) is used to access the infiltration of the skin and subcutaneous soft tissues. A hand-held Doppler ultrasound unit with a pencil probe or Smart needle (Peripheral Systems Group, Mountain View, CA) can be extremely helpful in obtaining vascular access in difficult cases. An initial injection of contrast through the needle or sheath should be used to confirm or rule out the presence of iliac or IVC thrombus in suspected cases. If thrombus is present, this access route should be abandoned in favor of the jugular vein approach to avoid dislodging the thrombus. If thrombus is not visualized, a floppy-tipped soft Bentson guidewire (Cook) is advanced into the IVC. A 5F pigtail catheter is passed over the guidewire and positioned in the right common iliac vein just below the IVC bifurcation. To avoid missing important IVC anomalies, some investigators advocate performing inferior venacavography from the left common femoral vein or placing the pigtail in the left common iliac vein when a jugular approach is used. The alternative would be to place the pigtail in the left common iliac vein from a right common femoral vein approach. Digital imaging with a marker catheter is used so that accurate measurements of the caval diameter (at the level of the renal veins) can be obtained. An injection rate of 15 mL of half-strength contrast per second for 2 seconds is adequate digital imaging. Filming is at two frames per second. Patients should be instructed to perform the Valsalva maneuver during the venacavogram to obtain maximum caval distention. If the caval diameter is greater than the maximum base diameter of the chosen filter, the legs will not engage the caval wall and the filter may migrate to the iliac veins, renal veins, heart, or pulmonary circulation.11,23 After filter placement, a follow-up (digital subtraction is adequate) venacavogram is helpful to document the filter’s position with respect to the IVC and its tributaries and for comparison in later cases of suspected IVC thrombosis or perforation. An image with bony landmarks is helpful as a baseline for comparison if suspected filter migration or tilting is suspected later. IVC Anomalies The normal right-sided IVC is derived from persistence of the embryonic right supracardinal vein. IVC duplication occurs when the embryonic right and left supracardinal veins persist. Both IVCs may be equal in size, but the right is usually larger (Fig. 39–1). The left IVC usually terminates at the left renal vein. The frequency of this anomaly is between 0.2 and 3%.24 Its significance lies in that placement of a filter in the “normal” right IVC will not prevent contralateral pelvic and lower-extremity emboli from reaching the lungs (through the left renal vein and suprarenal right IVC). Patients with this anomaly typically require filters in both IVCs.25,26 Embolization of the duplicated left IVC with filter placement in the right IVC has been reported as an alternative when the left IVC is significantly smaller than the right.27 A left-sided IVC occurs with persistence of the left supracardinal vein and obliteration of the right supracardinal vein. This anomaly occurs with a frequency of 0.2 to 5%.26 The left IVC drains the left renal vein, crosses over midline, and continues in the expected position of the normal renal and suprarenal segments of the IVC. Patients with this anomaly most likely should not undergo placement of the less flexible filters (i.e., Greenfield, Vena Tech) from a right common femoral vein or jugular vein approach, but rather from the left common femoral vein. The Bird’s Nest and Simon Nitinol filters, although easier placed from a left common femoral vein approach, have the flexibility in filter and delivery systems to undergo placement from a right-sided approach, if necessary. A circumaortic left renal vein occurs when there is persistence of the infrarenal segment of the left supracardinal vein (Fig. 39–2). This segment passes dorsum to the aorta and can cross the midline at the level of the left renal vein (aortic collar or two to three vertebral bodies below).27 The frequency of this anomaly is 1.5 to 8.7%.29,30 The anterior portion of the ring joins the IVC in the normal position expected of a left renal vein. The posterior portion of the ring follows a downward course posterior to the aorta until joining the IVC at a lower level. This anomaly requires that the filter be placed below the level of the insertion of the posterior portion of the left renal vein ring into the IVC. If not recognized at inferior venacavography, this segment can function as a collateral pathway for emboli to circumvent the filter and reach the lungs. Accessory renal veins may also occur, necessitating filter placement inferior to the main renal veins (Figs. 39–3 and 39–4). FIGURE 39–1. Duplication of the inferior vena cava (IVC). Patient with chronic renal failure and right femoral dialysis catheter had an inferior venacavogram performed before IVC–femoral vein dialysis fistula creation. The left IVC is slightly larger than the right IVC. Note the thrombus (arrow) at the right IVC–right common femoral vein junction. Suprarenal Filter Placement Suprarenal IVC Filter Placement Placement of an IVC filter above the level of the renal veins is indicated in cases of (1) renal vein thrombosis, (2) IVC thrombosis extending above the level of the renal veins (including thrombus secondary to tumor such as renal cell carcinoma) (Fig. 39–5), (3) filter placement during pregnancy and in women anticipating pregnancy, (4) recurrent pulmonary embolism after placement of an infrarenal filter in whom an upper-extremity source has been ruled out, and (5) pulmonary embolism following ovarian vein thrombosis. Few studies in the literature have evaluated suprarenal filter placement. A recent small retrospective study by Matchett et al31 suggested that suprarenal IVC filter placement is safe without any risk of associated permanent renal dysfunction.31 FIGURE 39–2. Circumaortic left renal vein, (a) The inferior segment (arrow) extends downward and posterior to the aorta prior to joining the IVC (arrowhead), (b) The superior segment (short arrow) passes anterior to the aorta prior to joining the IVC (arrowheads). Note that in this case the superior segment is significantly smaller than the posterior segment (long arrow). FIGURE 39–3. (a) A small wisp of contrast (arrow) was seen on the initial venacavogram. (b) This proved to be a low insertion of an accessory right renal vein (arrow). FIGURE 39–4. An inferior venacavogram performed from a tansjugular approach clearly identifies two (arrows) right renal veins. A Vena-Tech filter (arrowhead) was placed below the level of the inferior renal vein. Note the clot (curved arrow) extending interiorly from the base of the filter. FIGURE 39–5. Patient with metastatic ovarian carcinoma, (a) Inferior venacavogram shows large thrombus (arrows) extending above the level of the left renal vein (open arrow), (b) A Vena-Tech filter (arrow) was placed above the level of the thrombus from a transjugular approach. Superior Vena Caval Filter Placement Placement of a filter into the superior vena cava is indicated in cases of pulmonary embolism from an upper extremity or great thoracic vein source in patients who either have contraindications to or who have failed anticoagulation therapy. The perfect IVC filter should satisfy the following requirements: (1) low profile; (2) easy insertion; (3) high biocompatibility; (4) high durability, elasticity, and fatigue resistance; (5) noncorrosive; (6) nonthrombogenic; (7) nonferromagnetic; (8) retrievable (after a short insertion period); (9) 100% caval patency rate; (10) 0% recurrent pulmonary embolism rate; and (11) 0% caval perforation and migration rate. Although criteria 1 through 7 were obtained from an engineering standpoint, criteria 8 through 11 have proven much harder to accomplish. The filters presently available on the U.S. market all have reported caval patency rates greater than 90% and recurrent pulmonary embolism rates less than 10% in at least one or more studies. FIGURE 39–6. Mobin-Uddin umbrella filter (for transjugular approach) (a) viewed from above (courtesy of Kari Mobin-Uddin, M.D.), and (b) from the side (courtesy of Scott O. Trerotola, M.D.). For most radiologists, filter choice is thus a matter of personal preference, often based on training and filter availability. Vascular access and caval diameter are factors that do require an informed decision. Some filters are not capable, or at least not recommended by the manufacturer, to be placed from certain vascular approaches. For example, the preferred access for the 24F Greenfield filter is the right internal jugular vein or right common femoral vein, although the left internal jugular vein is not uncommonly used.12 Mediastinal bleeding and chest discomfort have been reported after the left internal jugular vein has been used for placement.32 Until the recent modifications of the delivery sheath, it was recommended by the manufacturer that the 12F titanium Greenfield filter not be placed from a left common femoral vein approach. Only the 7F Nitinol IVC filter reportedly can be placed from a left external jugular vein or antecubital vein approach.33,34 Caval diameter is a second limiting factor. All the available filters on the U.S. market except the Bird’s Nest IVC filter are limited to placement in patients with a corrected caval diameter of 28 mm or less. The 3% of patients with a caval diameter greater than 28 mm,35 but less than 48 mm, will require placement of either a Bird’s Nest IVC filter or bilateral common iliac vein filters. Mobin-Uddin Filter The Mobin-Uddin (MU) IVC filter (Fig. 39–6) was introduced as the first true intravascular inferior vena caval filter in 1967 by American Edwards Laboratories. It was released for general clinical use in January 1970. Originally designed for placement through a surgical venotomy, percutaneous methods for introduction were developed by 1973.36 Although the filter was extremely effective in preventing pulmonary embolism (recurrent pulmonary embolism rate, 3%; fatal pulmonary embolism rate, 0.8%), the IVC thrombosis and lower-extremity chronic venous stasis sequelae rates prove to be too high to support its continued use (Fig. 39–7). More than 10,000 MU filters were implanted before voluntary removal from the market by American Edwards Laboratories in December 1986. Stainless Steel Greenfield IVC Filter The 24F Kimray-Greenfield filter was introduced in 1973 (Fig. 39–8).37 Originally designed for insertion through a surgical venotomy, percutaneous insertion was not popularized until the 1980s.38 The filter is constructed of medical grade 316 L stainless steel with six zigzag legs radiating from a central hub at a 35-degree angle. The legs are 6 mm apart at the base of the filter and 2 mm apart at the hub. The distal end of each leg is turned upward 180 degrees to form a hook for stabilization in the caval wall. The filter is conical and has a height of 46 mm and a base diameter of 30 mm. The base diameter and radial force of expansion allow for placement in a vena cava 28 mm in diameter or less. The filter is loaded into a 24F carrier, which is passed through a 29.5F delivery sheath during surgical placement or a 24F vascular sheath during radiologic placement. The filter’s shape allows for clot trapping in the central portion with a high degree of efficiency (89 to 100%, depending on the size of the emboli), with maintenance of laminar blood flow around the periphery of the inside and outside of the cone,39 which results in a bathing of the trapped clot with flowing blood and promotes lysis of the trapped emboli. The conical shape of the filter preserves 51% of the cross-sectional diameter for filtering with 70% of the cone filled with thrombus.40 FIGURE 39–7. (a) Patient with Mobin-Uddin umbrella filter (arrow) and lower extremity chronic venous stasis sequela. The large vessel appears to be a patent IVC. (b) An oblique view shows that this vessel is a dilated azygos vein (arrows). The IVC is occluded. The 24F Greenfield filter has been evaluated extensively in clinical trials, and after 20 years on the market, numerous studies attesting to its efficiency have been published. The recurrent pulmonary embolism and IVC patency rates have ranged from 2 to 5% and 92 to 98%, respectively.10,12,41 Despite the fact that these figures for recurrent pulmonary embolism and caval patency rates are generally accepted (placing the 24F Greenfield filter in a position as the gold standard by which all other filters have been compared), closer examination of these studies is warranted.

 Indications for Inferior Vena Caval Filters
Indications for Inferior Vena Caval Filters
 Contraindications
Contraindications
 Patient Preparation
Patient Preparation
 Venography
Venography
 Choosing an Inferior Vena Caval Filter
Choosing an Inferior Vena Caval Filter
 Inferior Vena Caval Filters
Inferior Vena Caval Filters
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree