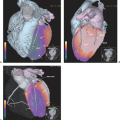
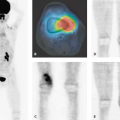
PET and PET-CT in Multiple Myeloma
Sven N. Reske
Anja Dankerl
Multiple myeloma (MM) represents a malignant clonal proliferation of plasma cells and commonly results in an overproduction of monoclonal immunoglobulins. Clinical presentation is usually associated with lytic bone lesions; extramedullary involvement mainly occurs in relapsing disease and may be attributed to aggressive illness. Diagnosis includes various blood and urine tests as well as radiographic bone survey, computed tomography (CT), and magnetic resonance imaging (MRI). Functional imaging with fluorodeoxyglucose (FDG) positron emission tomography (PET), best performed with CT-coregistration, is able to detect active myeloma and can be helpful in differentiating between posttherapeutic changes and residual or recurrent MM manifestations. Persistent findings after induction therapy predict early relapse. FDG-PET results were especially helpful in identifying focal recurrent disease in patients with nonsecretory or hyposecretory disease. Because of the increased synthesis of immunoglobulin in MM, amino acid use provides a new approach for functional imaging. Bone marrow uptake in patients with Durie/Salmon stages II to III was increased compared with homogenous and low uptake in controls. All patients with MM had [11C]methionine-positive osteolyses respective to extramedullary manifestations. Active MM displayed high [11C]methionine uptake. Based on these results, active MM could reliably be imaged with [11C]methionine PET-CT. Lesions with increased [11C]methionine uptake and normal bone structure suggest new metabolic active lesions developing earlier than structural osteolytic bone changes.
Introduction
Plasma cell neoplasms represent a spectrum of diseases characterized by clonal proliferation and accumulation of immunoglobulin (Ig)-producing terminally differentiated B cells. The spectrum includes clinically benign common conditions such as monoclonal gammopathy of unknown significance (MGUS) as well as rare disorders such as Castleman disease and γ heavy-chain disease; indolent conditions such as Waldenström macroglobulinemia (WM); the more common malignant entity plasma cell myeloma, a disseminated B-cell malignancy; and a more aggressive form, plasma cell leukemia, with circulating malignant plasma cells in the blood. All of these disorders share common features of plasma cell morphology, production of Ig molecules, and immune dysfunction. A plasma cell neoplasm is considered to originate from a single B cell, with resultant monoclonal protein secretion that characterizes its type (1,2).
Epidemiology
The incidence rate is 4 per 100,000 per year.
Approximately 15,000 new cases are diagnosed each year in the United States.
The current prevalence of myeloma in the United States is about 50,000, and 10,800 deaths from the disease were reported in 2001. Worldwide, it is estimated that there are at least 32,000 new cases reported and 24,000 deaths each year.
Myeloma is twice as common among African Americans as compared with whites and affects men more than women (2).
Clinical Manifestations
Patients with multiple myeloma (MM) may be entirely asymptomatic and diagnosed on routine blood testing or may present with a myriad of symptoms: hematologic manifestations, bone-related problems, infections, various organ dysfunctions, neurologic complaints, or bleeding tendencies. These signs and symptoms result from direct tumor involvement in bone marrow or extramedullary plasmacytomas, the effect of the protein produced by the tumor cells deposited in various organs, production of cytokines by the tumor cells or by the bone marrow microenvironment, and effects on the immune system.
Hypercalcemia and Bone Disease
The mechanism of bone abnormalities in myeloma, especially destruction, is an unbalanced process of increased osteoclast activity and suppressed osteoblast activity. These changes are due to an increase in osteoclast-activating factors produced predominantly by the bone marrow microenvironment but also by myeloma cells. As a result, osteoporosis and lytic bone lesions develop. These bone changes frequently involve the vertebral column and result in compression fractures, lytic bone lesions, and related pain. A new onset of back pain or other bone pain is a frequent presenting symptom in myeloma patients. Changes in the cytokine milieu and bone destruction may also lead to development of hypercalcemia, which is observed in approximately 25% of patients at some stage of the disease. Symptoms of high calcium levels include mental status changes, lethargy, constipation, and vomiting.
Extramedullary Disease
Extramedullary disease manifestations are uncommon in patients with myeloma at presentation. However, such manifestations have been observed in the setting of advanced-stage disease or relapse after allogeneic transplantation. Solitary or multiple extramedullary plasmacytomas have been described in the liver, spleen, lymph nodes, kidneys, subcutaneous tissues, and brain parenchyma. Extramedullary involvement may be suspected in patients who have more aggressive features of myeloma.
Diagnosis and Staging
Because myeloma patients present with various symptoms not specific to the disease, the diagnosis of myeloma is quite often delayed. An older patient with a new onset of unexplained back pain or bone pain, recurrent infection, anemia, or renal insufficiency should be screened for myeloma. Additional findings such as hyperproteinemia or proteinuria, anemia, hypoalbuminemia, low Ig levels, or marked elevation of erythrocyte sedimentation rate should prompt a further complete evaluation for diagnosis of plasma cell myeloma.
Table 56.1 Durie/Salmon Plus Staging Systema | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree