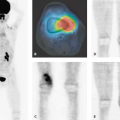
PET and PET-CT in Soft Tissue Sarcomas
Janet F. Eary
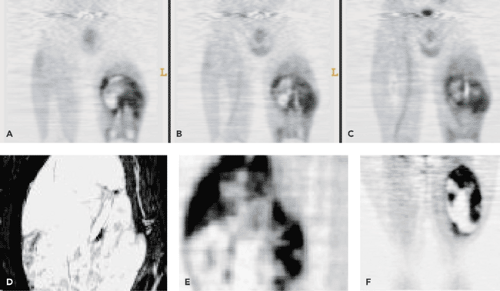
Soft tissue sarcomas are a complex group of neoplasms that are derived from mesenchymal tissue elements. They most commonly occur in the muscles and connective tissues. They provide treatment challenges because a tumor can present with low-grade to benign behavior or intermediate and aggressive high-grade behavior. Patients with large intermediate- and high-grade tumors have relatively poor 5-year survival rates. Many groups treat patients with aggressive tumors with neoadjuvant chemotherapy to reduce tumor growth and reduce the onset of metastases (usually to the lungs). Because of the importance of tumor biologic assessment for patient treatment planning, which may involve chemotherapy, radiotherapy, and surgical resection, positron emission tomography (PET) imaging is beginning to play an important role in soft tissue sarcoma biologic assessment. Fluorodeoxyglucose (FDG) PET is being evaluated for the ability to grade tumors, assess treatment response, and restage patients with recurrent and metastatic disease. This technique has shown the ability to distinguish low- from high-grade tumors effectively. Additional studies show it to be an effective way to assess response to neoadjuvant chemotherapy. Changes in response to treatment have also been shown to be predictive of disease-free interval and overall survival. However, because of the many soft tissue sarcoma histologic types, more studies with FDG are needed to help provide further understanding of the FDG-PET imaging characteristics of tumors in each tumor subtype grouping. Other new PET imaging agents are aggressively being evaluated for their ability to provide additional risk stratification in these patients whose tumors are often refractory to treatment. Figure 53.1 shows several examples of FDG-PET scans of patients with sarcomas. These tumors show a wide variety of uptake patterns and levels of tumor metabolism.
Introduction
Soft tissue sarcomas are a heterogeneous group of tumors that originate from mesenchymal tissues. Although they are well characterized by high-resolution magnetic resonance (MR) and computed tomography (CT) imaging, clinically important issues such as tumor grade, stage, and response to treatment cannot completely be evaluated with anatomic imaging. The variety in soft tissue sarcoma characteristics, origin, and biologic behavior make them particularly interesting for molecular imaging techniques using positron emission tomography (PET). The challenges that these tumors pose in their diagnosis and management have contributed to the definition of the role of clinical PET in sarcoma and are a developing area of PET research. With limited effective chemotherapy and radiotherapy treatment schemes available for these patients, the current emphasis in management includes identifying high-risk patients and those who may have greater sensitivity to treatment. PET imaging has made a significant contribution to these patient management issues by providing important biologic information for an individual patient tumor.
Sarcoma Classification
Although considered to be rare tumors, soft tissue sarcomas occur at an incidence of 1 to 2 per 100,000 people in
the United States. They are derived from mesenchymal tissues, which are the body structures that compose the soft, nonorgan, nonepithelial tissues. These tissues are muscles, connective tissue, fat, peripheral nerves, and blood vessels. Sarcomas derived from these elements show differentiation features of these tissues and retain some characteristics of the tissue of origin. Skeletal muscle is derived from myoblasts, which contain muscle fibers composed of actin and myosin. Connective tissue is composed primarily of fibroblasts, fibril structures of collagen and elastin, and ground substance, or matrix. Fat tissues are of two types. White fat, composed of lipocytes, is primarily located in the subcutaneous tissues, mediastinum, retroperitoneum, and abdomen. Brown fat is located in the neck, mediastinum, interscapular area, axillae, and the perirenal region (Fig. 33.24). Brown fat cells have many mitochondria, vacuolated cytoplasm, and a nucleus in the central location. They have a distinct microscopic and functional imaging appearance. Peripheral nerves are composed of axons, fibroblasts, perineural cells, and Schwann cells. Perineural cells originate from fibroblasts, and the Schwann cells are thought to be derived from the neuroectoderm. Blood vessels are composed of endothelial cells, pericytes, smooth muscle cells, and glomus cells. In most of today’s tumor classifications, the histologic type is determined by the type of differentiation that the cell exhibits rather than its presumed origin. On biopsy specimens, histopathologic diagnosis consists of light microscopic examination, the use of special tissue element stains, and immunocytochemistry to help characterize the cell type and level of differentiation. A list of common soft tissue sarcoma histologic types and age group associations is shown in Table 53.1. Increasingly, gene expression profiles are being used to help classify and predict the biologic behavior of soft tissue tumors (1,2,3). Standard radiologic examination consists of plain x-ray films, CT, and MRI. Benign and malignant tumors are not often easily distinguished by anatomic imaging. However, certain features such as size, structure of apparent origin, heterogeneity in density, and calcification can provide important clues as to the probability of malignancy. Patient factors such as age and tumor location are also significant for other relationships to tumor type.
the United States. They are derived from mesenchymal tissues, which are the body structures that compose the soft, nonorgan, nonepithelial tissues. These tissues are muscles, connective tissue, fat, peripheral nerves, and blood vessels. Sarcomas derived from these elements show differentiation features of these tissues and retain some characteristics of the tissue of origin. Skeletal muscle is derived from myoblasts, which contain muscle fibers composed of actin and myosin. Connective tissue is composed primarily of fibroblasts, fibril structures of collagen and elastin, and ground substance, or matrix. Fat tissues are of two types. White fat, composed of lipocytes, is primarily located in the subcutaneous tissues, mediastinum, retroperitoneum, and abdomen. Brown fat is located in the neck, mediastinum, interscapular area, axillae, and the perirenal region (Fig. 33.24). Brown fat cells have many mitochondria, vacuolated cytoplasm, and a nucleus in the central location. They have a distinct microscopic and functional imaging appearance. Peripheral nerves are composed of axons, fibroblasts, perineural cells, and Schwann cells. Perineural cells originate from fibroblasts, and the Schwann cells are thought to be derived from the neuroectoderm. Blood vessels are composed of endothelial cells, pericytes, smooth muscle cells, and glomus cells. In most of today’s tumor classifications, the histologic type is determined by the type of differentiation that the cell exhibits rather than its presumed origin. On biopsy specimens, histopathologic diagnosis consists of light microscopic examination, the use of special tissue element stains, and immunocytochemistry to help characterize the cell type and level of differentiation. A list of common soft tissue sarcoma histologic types and age group associations is shown in Table 53.1. Increasingly, gene expression profiles are being used to help classify and predict the biologic behavior of soft tissue tumors (1,2,3). Standard radiologic examination consists of plain x-ray films, CT, and MRI. Benign and malignant tumors are not often easily distinguished by anatomic imaging. However, certain features such as size, structure of apparent origin, heterogeneity in density, and calcification can provide important clues as to the probability of malignancy. Patient factors such as age and tumor location are also significant for other relationships to tumor type.
Sixty percent of soft tissue sarcomas occur in the limbs. Most of these occur in the lower extremities deep to fascial tissues. They are less commonly found in superficial locations in areas such as the hand and foot. Deep soft tissue sarcomas can grow to large dimensions before coming to clinical attention. Presumably, large (>5 cm in any dimension) tumors are considered to be at least intermediate grade until subjected to further assessment, which includes biopsy.
Table 53.1 Common Soft Tissue Sarcoma Histologic Subtypes | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
Sarcoma Patient Evaluation
The assessment of patients with sarcoma consists of determining the stage and grade of the tumor as accurately as possible for treatment planning. Both provide prognostic information for risk of metastasis and local recurrence. In sarcoma, the tumor stage refers to the site, size, and histologic grade of the tumor. Currently there are several systems in use for sarcoma staging. Most commonly used is the American Joint Conference on Cancer (AJCC) system, which is based on the Tumor-Node-Metastasis (TNM) assessment (4). Tumor grading systems are designed to reflect the prognosis for the sarcoma patient. The most commonly used scheme for providing histologic diagnosis and tumor grade is the French system (FNCLCC) (5). In this system, some tumors have fixed grades, such as low grade, or rarely metastasizing, or variable grade based on histologic features. The FNCLCC diagnosis and grading system for soft tissue sarcomas is shown in Table 53.2. Additional clinical grading is performed by the clinician, which is best described by an assimilation of all the clinical features of the patient. These include patient age and overall health status, symptoms, biopsy quality and tumor histologic type, tumor size, location, grade, radiologic appearance, and more recently, pattern and level of FDG uptake. In many centers, this clinical assessment is used to make the decision
for neoadjuvant chemotherapy or radiotherapy versus proceeding directly to surgical resection. A typical set of treatment guidelines for soft tissue sarcomas is presented in Table 53.3. Local variations on these guidelines are quite common.
for neoadjuvant chemotherapy or radiotherapy versus proceeding directly to surgical resection. A typical set of treatment guidelines for soft tissue sarcomas is presented in Table 53.3. Local variations on these guidelines are quite common.
Table 53.2 Soft Tissue Sarcomas Listed by Tumor Grade (Fnclcc) | |
|---|---|
|
FDG-PET in Soft Tissue Sarcoma Diagnosis
The use of FDG-PET in sarcoma patient assessment has risen steadily with the growth of clinical PET practice in cancer imaging. Several articles have appeared that undertook
meta-analyses of the available literature on the use of FDG-PET in sarcoma patient management (6,7,8). Overall, these reviews indicate utility for FDG-PET in sarcoma patient management, but point out that prospective imaging studies with larger patient populations are needed to more definitively address this role. This has been a difficult task, as most authors do not have large series of these tumors with similar histologies to study. They have shown that FDG-PET is useful in detecting extent of disease and for identifying metastases. However, since sarcomas often show marked heterogeneity in cell type and FDG uptake spatial distribution, accurate diagnosis of tumor grade for treatment planning is often difficult. Many clinicians perform diagnostic biopsies in the outpatient clinic for convenience and as a means to reduce operating room use. However, these small-core biopsies incised in a surgically convenient location may not be reflective of the most biologically aggressive area of the tumor. The resulting diagnosis then can suffer from sampling error, and the working diagnosis for treatment planning can have an error in tumor grade. This error can result in a misapplication of neoadjuvant therapy. FDG-PET imaging of sarcoma for diagnosis provides several advantages. The entire body and the entire tumor volume is examined, so sites of disease are detected with a high level of sensitivity and correlation with anatomic imaging.
meta-analyses of the available literature on the use of FDG-PET in sarcoma patient management (6,7,8). Overall, these reviews indicate utility for FDG-PET in sarcoma patient management, but point out that prospective imaging studies with larger patient populations are needed to more definitively address this role. This has been a difficult task, as most authors do not have large series of these tumors with similar histologies to study. They have shown that FDG-PET is useful in detecting extent of disease and for identifying metastases. However, since sarcomas often show marked heterogeneity in cell type and FDG uptake spatial distribution, accurate diagnosis of tumor grade for treatment planning is often difficult. Many clinicians perform diagnostic biopsies in the outpatient clinic for convenience and as a means to reduce operating room use. However, these small-core biopsies incised in a surgically convenient location may not be reflective of the most biologically aggressive area of the tumor. The resulting diagnosis then can suffer from sampling error, and the working diagnosis for treatment planning can have an error in tumor grade. This error can result in a misapplication of neoadjuvant therapy. FDG-PET imaging of sarcoma for diagnosis provides several advantages. The entire body and the entire tumor volume is examined, so sites of disease are detected with a high level of sensitivity and correlation with anatomic imaging.
Table 53.3 Soft Tissue Sarcoma Treatment Practices | |
|---|---|
|
The presence of necrosis is a tumor feature that automatically indicates a high-grade tumor in sarcomas with variable grades (Table 53.3.) This deterioration is easily seen on FDG-PET images and is an important prognostic finding. Figure 53.1 shows several examples of the presence of heterogeneous tumor uptake with tumor necrosis in sarcomas. The exception to this visual finding is the presence
of tumor elements that show little metabolic activity such as cartilage, fibrous serous fluid, blood, or ground substance accumulation.
of tumor elements that show little metabolic activity such as cartilage, fibrous serous fluid, blood, or ground substance accumulation.
Assessment of tumor grade from the FDG-PET image is a related goal for diagnosis. It stands to reason that the most biologically aggressive region of the tumor is reflected by a regional increase in FDG uptake. Sarcoma behavior is dictated by the most biologically aggressive component. Consequently, this is the area most critical to biopsy for diagnosis. On histologic examination, these areas have increased cellularity and mitotic rate, and often have increased levels of specific markers of tumor aggressiveness. Folpe et al. (9) found that increased tumor standardized uptake value (SUV) was associated with histopathologic grade, cellularity, mitotic activity, maximum-intensity projection (MIB) labeling index, and P53 overexpression in a series of both soft tissue and bone sarcomas. These data lend support to the concept that FDG-PET images can be used effectively to guide biopsy. Hain et al. (10) imaged 20 patients with soft tissue masses and clinical presentations suggestive of malignancy with FDG. Tumor regions thought to be malignant were found to be representative of the most malignant regions of the rest of the tumor. Tumors that were found to be benign on resection did not show significant FDG uptake.
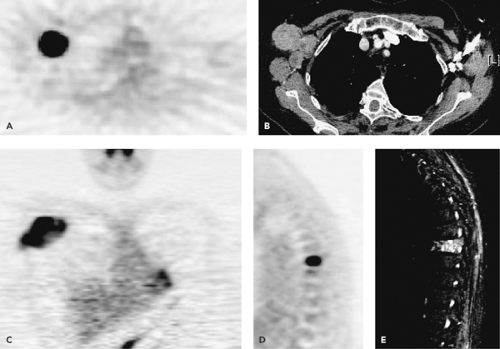
There are numerous published reports from investigators exploring the use of FDG-PET to grade sarcomas prior to treatment (11,12,13,14,15,16,17,18,19). These reports showed that there is a significant ability of the technique to distinguish benign or low-grade from high-grade tumors. Lucas et al. (17) found in a series of 30 patients that all high-grade tumors were correctly identified using qualitative image assessment, but ability to distinguish between a benign and a low-grade tumor was reduced. Using a quantitative assessment, they found a 95% sensitivity and a 75% specificity for diagnosis. This result is similar to the work published in other studies. Figures 53.2 and 53.3 show several examples of soft tissue sarcomas with different histologic types and tumor grades.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree