PET and SPECT-CT in Lymphoma
Karoline Spaepen
Sigrid Stroobants
Positron emission tomography (PET) using fluorodeoxyglucose (FDG) is now widely used for staging and monitoring treatment results in patients with Hodgkin disease (HD) and non-Hodgkin lymphoma (NHL). PET provides information complementary to conventional staging procedures for the staging of patients with HD and aggressive NHL. It is unlikely, however, that PET will fully replace conventional staging procedures in the staging of lymphoma patients. Moreover, the increasing use of integrated PET/computed tomography (CT) systems will probably result in a significant improvement in the diagnostic accuracy of both systems and will change the discussion to another level. The use of FDG-PET in low-grade lymphoma remains controversial. The most important current indication for PET is evaluation at the end of treatment. All patients with residual masses on conventional staging procedures should undergo FDG-PET to differentiate residual tissue from necrotic tissue. If PET is positive, before starting salvage therapy, a close correlation with clinical data, other imaging modalities, and/or biopsy is mandatory to exclude false-positive uptake. Patients with a negative PET have a good prognosis, but unfortunately, PET cannot exclude minimal residual disease, possibly leading to a later relapse. Promising data indicate that PET may be useful to identify early treatment failure after a few cycles of chemotherapy and before stem cell transplantation.
Introduction
Positron emission tomography (PET) using fluorodeoxyglucose (FDG) is now widely used for staging and monitoring treatment results in patients with Hodgkin disease (HD) and non-Hodgkin lymphoma (NHL). Together, HD and NHL constitute only 8% of all malignancies, but most patients are young and potentially curable. In contrast to many solid tumors, lymphomas are highly sensitive to chemotherapy or radiotherapy, and substantial long-term cure rates of 90% for HD and 50% for aggressive NHL are expected with the current treatment options. However, the magnitude of late treatment–related morbidity and mortality, especially in young HD patients treated with combination chemo-radiotherapy, and the fact that many NHL patients still cannot be cured with standard induction therapy, has tempered the initial enthusiasm. Accordingly, tailoring the intensity of the treatment to individual patients has become very important. FDG-PET has potential advantages to optimize the management of lymphoma patients in three principal domains: improving the accuracy of initial staging, assessing the response to treatment earlier and more accurately, and optimizing the follow-up after therapy. In this chapter, the value of PET in these different areas is discussed.
Initial Staging
Until recently, staging of lymphoma was done by ultrasound (US), computed tomography (CT), or magnetic resonance imaging (MRI). Although these imaging tools allow exquisite anatomic detail, in essence they all require alterations of anatomic structures to suggest tumor. Minimally
affected lymph nodes of normal size or parenchymal involvement with insufficient contrast to surrounding tissue may therefore be missed, whereas inflammatory enlarged lymph nodes may be erroneously interpreted as representing tumor deposits. With regard to bone marrow involvement, iliac crest biopsy is performed routinely, but because of the frequently patchy involvement, lesions can be missed with this focal approach. Therefore, there has always been a strong need for a sensitive noninvasive imaging technique that uses other than anatomic characteristics of tissue to detect tumor activity.
affected lymph nodes of normal size or parenchymal involvement with insufficient contrast to surrounding tissue may therefore be missed, whereas inflammatory enlarged lymph nodes may be erroneously interpreted as representing tumor deposits. With regard to bone marrow involvement, iliac crest biopsy is performed routinely, but because of the frequently patchy involvement, lesions can be missed with this focal approach. Therefore, there has always been a strong need for a sensitive noninvasive imaging technique that uses other than anatomic characteristics of tissue to detect tumor activity.
The first imaging tool largely independent of morphologic criteria was 67gallium single-photon emission computed tomography (67Ga-SPECT). This technique was routinely used for the evaluation of residual masses after chemotherapy in lymphoma patients and has been shown to be very useful in the monitoring of disease response (1,2,3). Soon, 67Ga-SPECT became a standard method for therapy assessment. In 1997, Paul (4) reported on the use of FDG-PET for the detection of lymphomas compared with 67Ga and stated that FDG was superior to 67Ga for the detection of lymphoma. This prompted more extensive research into the role of FDG-PET for the staging of lymphoma patients. In comparison with 67Ga-SPECT, FDG-PET seems to be the favorable technique because of the superior resolution and sensitivity of PET imaging methods together with better interpretability of abdominal findings, the lower radiation burden (7 mSv for FDG-PET versus 37 mSv for 67Ga-SPECT), and the shorter examination time (2 hours for FDG-PET versus 3 days for 67Ga-SPECT).
Over the last decade, numerous articles have appeared concerning the role of FDG-PET in initial staging of lymphoma patients (5). Because FDG-PET has been introduced into the clinical environment without randomized trials or rigorous testing that required the investigator and patients to be blinded to the results of a new imaging modality, its precise role in disease staging has not been well defined. Moreover, all published data suffer from methodologic problems inherent to lymphoma imaging studies because a biopsy was obtained for only a few lymph nodes. For ethical reasons, previously unknown lesions detected by PET were sampled only when the results potentially influenced staging and treatment. The calculation of sensitivity and specificity is not possible in the absence of histologic proof. The methodology used in nearly all studies is mostly in favor of imaging techniques with high sensitivity and low specificity (6). Nevertheless, despite these limitations, in a recent meta-analysis (7) on the use of FDG-PET for initial staging of lymphoma that included 20 eligible studies between 1995 and 2004, the pooled sensitivity was 90.9% and the pooled false-positive rate was 10.3% for FDG-PET in initial staging. The pooled sensitivity and false-positive rate appeared to be higher in patients with HD compared with those with NHL. These latter findings should be interpreted with caution because they are based on a small number of patients. Conversely, NHL is a highly heterogeneous disease that includes a large series of different entities; therefore, the diagnostic accuracy of FDG-PET may differ within the group of patients with NHL. Although most studies investigated both HD and NHL together, separate analyses are mandatory since staging results may lead to different patient management and outcome in both groups.
Hodgkin Disease
Several studies have investigated the role of FDG-PET in lymph node staging. Clinical findings, ultrasound (US), CT, MRI, and iliac crest biopsy have been compared. Moog et al. (8) studied discordant findings between FDG-PET and CT and demonstrated that disease was present in areas of increased FDG uptake and that there was no evidence of disease in enlarged nodes on CT when FDG was negative. Bangerter et al. (9) studied 44 patients with newly diagnosed HD. FDG-PET was positive in 38 of 44 patients (86%) at sites of documented active disease. Unfortunately, in six other cases PET either failed to visualize sites of HD (n = 4) or indicated a false-positive result (n = 2). A limitation of this study is that not all positive FDG-PET results were confirmed by biopsy and that some PET results may have been incorrectly interpreted as malignant. Jerusalem et al. (10) evaluated the role of FDG-PET compared to routine procedures for staging in 33 patients with HD. PET results were confirmed by biopsy only if they had an impact on patient management. The sensitivity of FDG-PET to detect all known pathologic lymph nodes was 83% for peripheral lymph nodes, 91% for thoracic lymph nodes, and 75% for abdominal and pelvic lymph nodes. PET detected focal liver infiltration in only one patient. Weihrauch et al. (11) studied 22 HD patients in whom 77 lesions were detected by FDG-PET, CT, or both. In 48 lesions (62%), the results were concordant; in 20 (26%), lesions only PET was positive; and in 9 lesions, only CT was positive.
Overall, there is little doubt that FDG-PET detects more lesions than CT and also more active disease in lesions that are not positive on CT based on size criteria. But, it is not certain that FDG-PET detects all disease, and it is difficult to calculate the false-positive rate with FDG-PET from literature. Moreover, the potential impact of false-positive or false-negative PET findings on treatment strategy has not been reported. Organ involvement in newly diagnosed HD, specifically the spleen, was studied by Rini et al. (12). In 32 patients, they found that FDG-PET was more accurate than 67Ga scintigraphy: it was twice as sensitive with similar specificity. Some studies also assessed the accuracy in detection of bone marrow involvement in comparison with iliac crest biopsies. Carr et al. (13) reported that FDG-PET can correctly assess bone marrow disease status in a high proportion of patients and has the potential to reduce the need for bone marrow biopsy. Twelve HD patients underwent FDG-PET and unilateral iliac crest marrow aspirates and biopsies. Two cases were concordantly positive, six were concordantly negative, and in four patients the FDG-PET scan was positive and biopsy negative. Subsequent biopsy
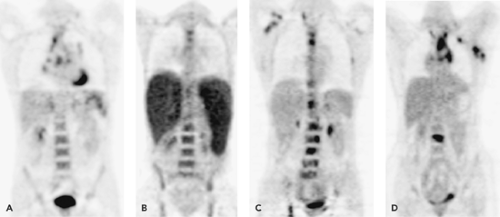
in one confirmed BM involvement, two cases were confirmed to be false-negative, and one remained equivocal. In the two false-positive patients, reactive myeloid hyperplasia characteristic of some HD patients may explain increased FDG uptake. From this and other studies (14), it can be concluded that FDG-PET scanning may improve the sensitivity of bone marrow disease detection in HD over blind bone marrow biopsy, in the sense that it can guide the localization where the biopsy should be preferentially taken (Fig. 55.1).
in one confirmed BM involvement, two cases were confirmed to be false-negative, and one remained equivocal. In the two false-positive patients, reactive myeloid hyperplasia characteristic of some HD patients may explain increased FDG uptake. From this and other studies (14), it can be concluded that FDG-PET scanning may improve the sensitivity of bone marrow disease detection in HD over blind bone marrow biopsy, in the sense that it can guide the localization where the biopsy should be preferentially taken (Fig. 55.1).
A more relevant question is how FDG-PET affects the staging of primary lymphoma and hence patient management. Bangerter et al. (9) detected unknown lesions in five patients; one patient was up-staged from stage I to II, three patients from stage II to IV, and one patient from stage III to IV. In another patient, PET indicated a down-staging from stage II to I. As a result of changing stage, treatment strategy had to be changed in each case (6/44 = 14%). Similarly, Partridge et al. (15) retrospectively compared CT (chest, abdomen, and pelvis) and FDG-PET in the staging of 44 patients with HD. Up-staging by PET was reported in 18 of 44 patients (40.9%) and down-staging in 3 of 44 patients (6.8%). In a study by Weihrauch et al. (11), staging was altered in 4 of 22 patients based on PET. In contrast to these studies, in which in most cases there was an up-staging based on PET, Jerusalem et al. (10) reported down-staging in 4 of 33 patients, including a biopsy-proven case. However, one of these cases was down-staged in error. PET suggested an up-staging in three patients. Also, the study from Hueltenschmidt et al. (16) showed down-staging in 7 of 25 patients based on FDG-PET and up-staging in only 3 patients compared with conventional staging procedures: 1 from stage I to IV and 2 from stage II to III.
The main issue is whether a change in disease stage alters the therapy management of the patient. This was the case in all patients of Bangerter, in half of the patients in the study of Partridge, but in only two patients in the study of Hueltenschmidt and in one case in the two other studies (Jerusalem and Weihrauch). Apparently, differences in study design, whether the study was conducted in a primary or tertiary care center, whether it was a single or multicenter study, and the timepoint enormously affected the final decision making. In general, good-risk localized patients (stage IA and IIA) are treated with a limited course of chemotherapy and field radiotherapy whereas advanced-stage HD patients receive several courses of combination chemotherapy. So a stage change from IA to IIA may lead to a change in radiotherapy field and a change from IIA to IIIA may influence the course of chemotherapy. Other changes will not affect the patient management. This issue was addressed in by Naumann et al. (17). They analyzed the therapeutic relevance according to the initial stage of patients and found the highest impact in early-stage HD with treatment intensification in 20% of the patients. Whether or not a change in therapy translates to an improved survival has not yet been fully addressed. In a recent study of Munker et al. (18), the contribution of PET to the prognosis of patients with HD was investigated. Of the 73 patients, 21 patients (28.8%) were up-staged by FDG-PET. If only early-stage patients and major stage
changes are considered (stage IA–IIB to III–IV), among 49 patients, 10 were up-staged to III or IV, whereas in 39 patients, staging was unchanged following PET. Interestingly, in the former group, three relapsed or were refractory compared to none in the latter group (p < 0.006). In advanced-stage patients, a trend toward treatment failure was apparent in patients who were up-staged by PET. It must be noted, however, that a change of treatment may ultimately not result in improved survival; as for HD, many patients can be cured with salvage therapy. From all the available literature, it appears that FDG-PET in HD improves the accuracy of staging, but in the absence of evidence that this leads to survival benefits, the high cost of FDG-PET remains an important issue and cost-benefit studies are warranted.
changes are considered (stage IA–IIB to III–IV), among 49 patients, 10 were up-staged to III or IV, whereas in 39 patients, staging was unchanged following PET. Interestingly, in the former group, three relapsed or were refractory compared to none in the latter group (p < 0.006). In advanced-stage patients, a trend toward treatment failure was apparent in patients who were up-staged by PET. It must be noted, however, that a change of treatment may ultimately not result in improved survival; as for HD, many patients can be cured with salvage therapy. From all the available literature, it appears that FDG-PET in HD improves the accuracy of staging, but in the absence of evidence that this leads to survival benefits, the high cost of FDG-PET remains an important issue and cost-benefit studies are warranted.
Non-Hodgkin Lymphoma
Most studies using FDG-PET have been conducted in NHL patients. From the beginning, authors have suggested that the accuracy of FDG-PET depends on histologic subtype. For aggressive NHL, FDG-PET is accepted as a useful tool for staging, but for indolent NHL, the results are very confounding, and fewer studies have appeared on this matter.
Indolent NHL
The FDG uptake in lymphoma patients is correlated to histologic grade and proliferative activity. In a recent large study, Schöder et al. (19) concluded that the standardized uptake value (SUV) is lower for indolent lymphoma than for aggressive lymphoma, that patients with a SUV greater than 10 have a high likelihood of aggressive lymphoma compared with those with a SUV less than 6, which is likely associated with indolent lymphoma. These results are similar to other previously reported smaller studies (20,21,22).
Only limited and conflicting data have been published about the sensitivity of FDG-PET in low-grade NHL. Najjar et al. (23) reported sensitivity and specificity for FDG-PET of 87% and 100%, respectively, in 36 indolent lymphoma patients, and Wirth et al. (24) reported PET-positive findings of 90%. In contrast to these studies, Jerusalem et al. (25) studied 36 patients with low-grade NHL. PET detected 40% more abnormal lymph node areas than conventional staging in follicular lymphoma, but was inappropriate for the staging of small lymphocytic lymphoma for which it detected less than 58% of abnormal lymph node areas. The number of patients with mucosa-associated lymphoid tissue (MALT) or mantle cell lymphoma was too low to permit any conclusion. The location of nodal lesions was important: PET showed more lesions than conventional staging for peripheral (34% more lymph node areas detected) and thoracic lymph nodes (39% more lymph node areas detected) but not for abdominal or pelvic lymph nodes (26% fewer areas detected). The sensitivity to detect bone marrow infiltration was unacceptably low regardless of the histologic subtype. In contrast, PET was as effective as standard procedures for the detection of other extranodal localizations, although a few localizations were detected only by PET and a few others only by conventional procedures. Overall, because of the frequent false-negativity of FDG-PET and the general lack of impact of stage alone on the therapy given, the evidence for including FDG-PET in the routine diagnostic staging of indolent lymphoma is not strong. Further studies are warranted to see whether FDG-PET can be used in the staging of some subtypes of indolent lymphoma that probably express sufficient FDG avidity.
Aggressive NHL
Elstrom et al. (26) studied 70 patients with aggressive NHL and correlated the results with the pathologic diagnosis. Disease was detected in 100% of patients with diffuse large B-cell lymphoma and mantle cell lymphoma. In contrast, only 40% of peripheral T-cell NHL were detected, suggesting that FDG-PET is more useful for B-cell than T-cell lymphoma. In the same study, anaplastic large cell and Burkitt’s lymphoma also could be detected. Most authors (27,28,29,30,31,32) studied patients with both HD and NHL of various histologic subtypes and compared the FDG-PET results with those of CT or 67gallium scanning in a lesion-based analysis. The results are similar to those reported for HD-only patients in that FDG-PET visualizes more lesions than CT and surely is superior to 67Ga scintigraphy, but lack of histologic proof is a known methodologic problem. Also, as for HD, the role of FDG-PET in the assessment of bone marrow involvement in NHL is not yet fully established. The group from Liège (33) reported a study comprising only patients with histologically proven aggressive NHL. Forty patients were studied at initial diagnosis, and 13 patients underwent PET for restaging after disease recurrence. The results of PET were compared with the results of conventional staging including clinical examination; CT of the thorax, abdomen, and pelvis; and bone marrow biopsy. PET identified 39 clinically undetected lymph node areas, whereas clinical examination showed only 9 additional lesions not seen by PET. Fifty-nine lymph node areas were detected by PET and clinical examination. PET identified 21 thoracic and abdominopelvic lymph node regions not seen by CT, whereas CT showed only 5 areas not demonstrated by PET. Both imaging techniques showed the same 56 areas of lymph node infiltration in the thorax, abdomen, or pelvis. The results suggested that FDG-PET is more sensitive than clinical examination and CT for the detection of lymph node regions infiltrated by NHL. PET is as effective as CT for the detection of extranodal disease excluding bone marrow. PET and CT identified the same 47 lesions, whereas 6 lesions were identified only by PET and 7 lesions were shown only by CT. PET missed known bone marrow infiltration in five patients. On the other hand, bone marrow infiltration suspected by PET was confirmed by biopsy in six of ten patients. The authors concluded that FDG-PET is an efficient, noninvasive method for staging and restaging aggressive NHL, but bone marrow biopsy still needs to be performed in addition to PET.
FDG-PET can replace bone scintigraphy in primary staging of malignant lymphoma (34). Osteolytic lesions, which are commonly observed in NHL, can escape scintigraphic detection. FDG-PET can identify osseous involvement with a high positive-predictive value and is more sensitive and specific than bone scintigraphy. Fifty-six patients (34 HD, 22 NHL) were studied by both imaging techniques. Bone scintigraphy showed skeletal involvement in 20 regions of 12 patients compared with PET, which showed 30 regions in the same patients. FDG-PET detected 12 additional regions in 5 other patients; these results were verified in 3 and unresolved in 2 patients. Conversely, bone scintigraphy identified five abnormalities in five other patients; the results were found to be erroneous in 3 and unresolved in 2 patients. FDG-PET may also be useful to differentiate primary CNS lymphoma from nonneoplastic lesions such as toxoplasmosis in patients with AIDS (35,36,37,38) (also see Chapter 24).
The key issue remains whether the increased detection of disease sites using FDG-PET alters staging and consequently the therapy given. In the study of Buchmann et al. (39), FDG-PET led to up-staging and change in therapy in only 4 of 52 patients. It should also be noted that in most centers NHL patients, in contrast to HD patients, receive the same therapy regardless of the stage at initial diagnosis. In these settings, the rationale for FDG-PET scanning is mainly to improve the evaluation of response assessment scans.
Use of Integrated PET-CT Systems
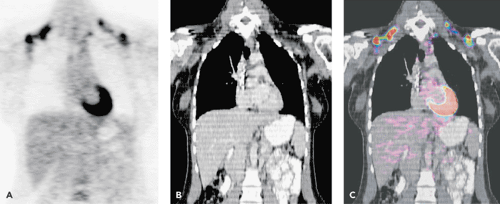
Isolated PET scanners are progressively being replaced by PET-CT systems, which combine a PET and a CT scanner in a single instrument, coregistering the anatomic information of CT with the functional images of PET. This approach can result in a significant improvement of the diagnostic accuracy of the PET findings, because the more accurate anatomic localization leads to fewer false-positive results owing to inter-patient variability in physiologic FDG uptake (Figs. 55.2 and 33.24). A study by Allen-Auerbach et al. (40) showed that PET-CT was superior to PET alone with reported accuracies of 93% and 84%, respectively. Freudenberg et al. (41) showed a sensitivity of 78% for CT alone, 86% for PET alone, and 93% for PET-CT. The clinical impact of combined PET-CT data was assessed by Raanani et al. (42) in 103 patients. The addition of PET-CT to CT changes the management decisions in approximately a quarter of NHL patients and a third of HD patients, mostly in early stages. It is likely that PET-CT will emerge as the pre-eminent tool in lymphoma imaging. Further studies are warranted to investigate whether intravenous contrast and whether high or low multi ampire seconds is mandatory.
Evaluation of Treatment Response
One of the most challenging aspects in lymphoma imaging is the assessment of response to treatment. The limitations of conventional imaging procedures are well known. Tumor volume reduction based on morphologic criteria is a late sign of effective therapy. Residual masses may be present at the end of treatment, even in patients responding very well, with normalization of all clinical and biologic abnormalities. Unfortunately, there are no reliable characteristics for CT that permit one to differentiate between malignant and fibrotic or necrotic tissue. MRI did not prove to be more
useful than CT for predicting the nature of such residual masses (43). Functional imaging modalities such as 67Ga scintigraphy and more recently FDG-PET provides information complementary to conventional imaging techniques.
useful than CT for predicting the nature of such residual masses (43). Functional imaging modalities such as 67Ga scintigraphy and more recently FDG-PET provides information complementary to conventional imaging techniques.
Evaluation of Residual Disease at the End of Treatment
Obtaining a complete remission (CR) after first-line chemotherapy is the main objective in lymphoma patients as it is usually associated with a longer progression-free survival (PFS). This is in contrast with a partial remission (PR), which is associated with a poorer clinical outcome (44). Approximately two thirds of patients with HD present with a residual mass, but less than 20% will ultimately relapse (45). Similarly, in NHL, residual tumor masses will be present in about 50% of patients with aggressive NHL, whereas only 25% of these patients will relapse (45). Therefore, many residual masses after completion of therapy essentially consist of fibrotic tissue. Since structural imaging cannot reliably discriminate fibrotic from viable tumor masses, international experts have tried to refine the criteria defining a complete response. Because of the uncertainty in the definition of a CR, a new category of response, “complete remission unconfirmed,” was created to reflect the unknown significance of persisting radiologic abnormalities in patients who seem to be otherwise in CR (46). Despite the introduction of high-resolution CT and despite the introduction of this new term used to describe the response to treatment, CT and MRI cannot reliably predict the clinical outcome after therapy. Early identification of patients who have not been cured by their primary treatment is important, however, because a better outcome can be expected after further treatment when such patients are treated at an earlier stage with lower tumor burden.
Functional imaging with 67Ga was for many years the imaging technique of choice to characterize residual masses after treatment (47). However, its sensitivity varies with the localization and size of the lesion (48). 67Ga scintigraphy is more accurate for evaluating masses in the mediastinum than in the abdomen, and sensitivity decreases for lesions smaller than 1.5 cm. Also, nonspecific uptake in the hilar regions and in the thymus of young patients with thymic rebound after chemotherapy can lead to false-positive findings. Since not all lymphomas take up 67Ga, a baseline study is necessary to prove gallium avidity.
Over the last years, 67Ga scintigraphy has been gradually replaced by FDG-PET, given its higher sensitivity and resolution and more favorable dosimetry. Several studies have shown the effectiveness of FDG-PET in the detection of residual disease at the end of treatment. Many of the early studies were performed on mixed lymphoma populations, including both NHL and HD. In a recent review (49), the results of 8 studies with mixed populations (174 HD and 183 NHL patients) were pooled, and a sensitivity of 79%, specificity of 94%, positive-predictive value (PPV) of 82%, and negative-predictive value (NPV) of 93% for PET at the end of treatment were calculated. The authors concluded that FDG-PET is the imaging technique of choice for the end-of-treatment evaluation in patients with lymphoma, but close correlation with clinical data, other imaging modalities, and/or biopsy are mandatory to exclude results that are either false-positive (inflammatory changes, FDG uptake in brown fat or muscle) or false-negative (minimal residual disease. HD and NHL are, however, two disease entities with clearly different histopathology, treatment, and prognosis, so results of PET should ideally be analyzed separately as was done in more recent publications.
Hodgkin Disease
HD is one of the few adult malignancies that in most instances can be successfully treated. Compared with patients with NHL, patients with HD are younger and present more often with stage I or II disease and are often treated with combination chemo-radiotherapy. However, late morbidity with cardiovascular toxicity and secondary malignancies are being increasingly recognized and this in turn has lead to question the standard use of radiotherapy for residual masses. Treatment in these patients should be limited to a minimum, without compromising the clinical outcome. The high negative-predictive value of FDG-PET can be of special interest to select patients with nonviable residual masses after chemotherapy in whom radiotherapy can be omitted. Table 55.1 presents an overview of different studies that report specifically on the value of PET at the end of treatment in HD (50,51,52,53,54,55,56,57,58,59). A high negative-predictive value of FDG-PET has been consistently reported by most studies and clearly identifies patients with an excellent prognosis. Relapses are infrequent and occur rarely within the first year after the end of treatment, probably reflecting minimal tumor burden below the detection limit of the PET system at the time of restaging. Since a substantial number of patients still receive additional radiotherapy (RT) after a negative PET scan, the reported NPV can, however, be overestimated. Furthermore, almost all patients with early-stage HD have a negative posttreatment PET and survive without relapse, probably reflecting the excellent prognosis of these patients more than the NPV of PET. The question of whether radiotherapy can be omitted in early-stage HD with a negative PET scan after first-line chemotherapy is therefore still unanswered. It is hoped that this will be resolved once we know the results of the ongoing randomized National Cancer Research Institute Phase III trial in the United Kingdom in which patients with clinical stage IA/IIA HD who are PET-negative after three cycles of ABVD (Adriamycin, bleomycin, vinblastine, and dacarbazine) are randomized between either involved field radiotherapy or no further treatment.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree