PET and SPECT in Other Cardiac Diseases
Frank M. Bengel
Markus Schwaiger
In several noncoronary cardiac diseases, nuclear imaging has been used for the noninvasive assessment of myocardial metabolism, microvascular reactivity, and autonomic innervation. The application of positron emission tomography (PET) and single-photon emission computed tomography (SPECT) has substantially contributed to our understanding of pathophysiologic relationships. In addition to their value as research tools, PET and SPECT may be helpful in the clinical setting, as some studies in noncoronary heart disease have indicated. In idiopathic dilated cardiomyopathy, prognosis may be determined by the identification of impairments of sympathetic innervation (Fig. 31.1) or microvascular function. The effects of therapeutic approaches on the tissue level can be monitored in cardiomyopathies. In the transplanted heart, nuclear imaging allows early identification of rejection and allograft vasculopathy and assessment of sympathetic reinnervation. Finally, in pediatric cardiology, evaluation of myocardial perfusion and viability may contribute to the workup of children who have Kawasaki disease or have undergone surgical coronary reimplantation due to congenital abnormalities.
Introduction
Compared to existing evidence in the diagnostic and prognostic workup of coronary artery disease, few data exist on clinical applications of nuclear imaging in noncoronary heart disease. However, imaging of myocardial perfusion, metabolism, innervation, and other molecular targets has substantially contributed to the characterization of specific aspects of the sometimes poorly understood pathophysiology of some noncoronary cardiac diseases. Based on an improved understanding of pathogenetic mechanisms, nuclear imaging approaches hold promise for the future evaluation of therapeutic effects and the prediction of outcome in these patients.
This chapter provides an overview of existing data for single-photon emission computed tomography (SPECT) and positron emission tomography (PET) imaging keyed to different types of noncoronary cardiac disease. Basic pathophysiologic research is outlined, but special emphasis is given to those approaches that have emerged as tools in clinical trials and that may thus contribute to patient workup in the future. Table 31.1 summarizes the different types of noncoronary diseases of the heart treated in this chapter, along with the specific nuclear imaging applications.
Idiopathic Dilated Cardiomyopathy
Idiopathic dilated cardiomyopathy can be separated from ischemic cardiomyopathy as the source of left ventricular dysfunction by use of nuclear imaging. In PET viability imaging, idiopathic cardiomyopathy shows more regional homogeneity and fewer and less severe defects in nitrogen
13 [13N]ammonia perfusion and fluorine 18 deoxyglucose (18F-FDG) metabolic images (1). Additionally, in gated myocardial perfusion SPECT imaging, the regional heterogeneity of wall motion abnormalities and perfusion is pronounced in ischemic cardiomyopathy (2).
13 [13N]ammonia perfusion and fluorine 18 deoxyglucose (18F-FDG) metabolic images (1). Additionally, in gated myocardial perfusion SPECT imaging, the regional heterogeneity of wall motion abnormalities and perfusion is pronounced in ischemic cardiomyopathy (2).
In addition to the relative regional perfusion homogeneity in PET and SPECT qualitative images, quantitative investigations of global myocardial blood flow by PET have revealed abnormalities of microcirculatory function in idiopathic dilated cardiomyopathy. In patients with normal coronaries, overt heart failure, and depressed left ventricular function, Merlet et al. demonstrated substantially reduced hyperemic flow in response to dipyridamole vasodilation compared with normals, which correlated with intracoronary Doppler flow measurements (3). Drzezga et al. additionally observed an impaired flow response to sympathetic stimulation using the cold pressor test (4). Neglia et al. investigated cardiomyopathy patients without overt heart failure, and found impaired blood flow both at rest and during pharmacologic vasodilation, suggesting that abnormalities can be present without severe alterations of hemodynamics. Progression of disease was associated with more severe depression of perfusion in this study (5). Furthermore, the same group identified depressed blood flow during pharmacologic vasodilation as a predictor of adverse outcome in a more recent study (6). It remains unclear, however, whether the observed alterations of myocardial blood flow are part of the still unknown pathophysiology of idiopathic dilated cardiomyopathy or whether they are epiphenomena.
An innovative approach to characterizing heart failure in cardiomyopathy is to combine the assessment of overall oxidative metabolism using carbon 11 [11C]acetate PET (Fig. 31.2) with noninvasive measures of ventricular function and cardiac work. Myocardial efficiency, which is defined by the amount of oxidative energy required to maintain a given level of cardiac work, can then be estimated by noninvasive means (Fig. 31.3). As might be expected, PET-derived estimates of efficiency are reduced in dilated cardiomyopathy compared with normals (7). More importantly, this approach can be used for a detailed evaluation of therapy effects by taking into account the energy cost of an increase of cardiac output achieved by a therapeutic measure. Beneficial effects of afterload reduction (8), dobutamine (9), β-adrenoceptor blockade (10), and resynchronization therapy (11) on cardiac efficiency have already been demonstrated. Noninvasive estimation of myocardial efficiency thus represents an appealing endpoint in clinical trials and may even serve to optimize individual therapy in the future.
Alterations of the sympathetic nervous system are a common feature of progressive heart failure. Sympathetic
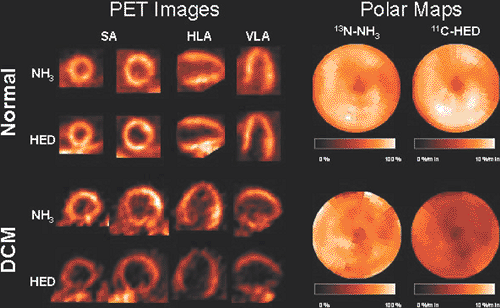
activation results in elevation of systemic catecholamine levels and subsequent down-regulation of postsynaptic β-adrenoceptors, as demonstrated by Merlet et al. using PET and the β-receptor ligand 11C CGP12177 (12). In addition, presynaptic alterations can be observed, as indicated by globally reduced myocardial uptake of the catecholamine analogs iodine 123 (123I) meta-iodobenzylguanidine (MIBG) imaged by SPECT or 11C hydroxyephedrine (HED) imaged by PET (see Fig. 31.1). In 29 patients, Hartmann et al. observed abnormally low HED retention in 64% ± 32% of the left ventricle despite nearly normal perfusion. The degree of presynaptic alterations was correlated with reduction of ejection fraction and New York Heart Association (NYHA) status (13). In a more recent study, a correlation with impaired metabolic efficiency was also observed (14). These PET-determined alterations of presynaptic sympathetic innervation thus seem to reflect the severity of heart failure in dilated cardiomyopathy and are in line with similar observations using SPECT and 123I-MIBG (15). Further observations in larger groups of patients showed that reduced presynaptic sympathetic activity identified by 123I-MIBG SPECT is associated with impaired outcome and represents an independent prognostic parameter (16). This prognostic value has been confirmed by Pietila et al. using HED PET (17). Finally, measures of presynaptic sympathetic impairment can be used to predict the response to medical therapy, such as β-blockade (18) and ACE inhibition (19).
activation results in elevation of systemic catecholamine levels and subsequent down-regulation of postsynaptic β-adrenoceptors, as demonstrated by Merlet et al. using PET and the β-receptor ligand 11C CGP12177 (12). In addition, presynaptic alterations can be observed, as indicated by globally reduced myocardial uptake of the catecholamine analogs iodine 123 (123I) meta-iodobenzylguanidine (MIBG) imaged by SPECT or 11C hydroxyephedrine (HED) imaged by PET (see Fig. 31.1). In 29 patients, Hartmann et al. observed abnormally low HED retention in 64% ± 32% of the left ventricle despite nearly normal perfusion. The degree of presynaptic alterations was correlated with reduction of ejection fraction and New York Heart Association (NYHA) status (13). In a more recent study, a correlation with impaired metabolic efficiency was also observed (14). These PET-determined alterations of presynaptic sympathetic innervation thus seem to reflect the severity of heart failure in dilated cardiomyopathy and are in line with similar observations using SPECT and 123I-MIBG (15). Further observations in larger groups of patients showed that reduced presynaptic sympathetic activity identified by 123I-MIBG SPECT is associated with impaired outcome and represents an independent prognostic parameter (16). This prognostic value has been confirmed by Pietila et al. using HED PET (17). Finally, measures of presynaptic sympathetic impairment can be used to predict the response to medical therapy, such as β-blockade (18) and ACE inhibition (19).
Table 31.1 SPECT and PET in Noncoronary Heart Disease | ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy is a mostly genetically determined disease and results in abnormal hypertrophy of the myocardium, which often is regionally heterogeneous and
especially pronounced in the septum. In patients symptomatic for chest pain, Nienaber et al. observed reduced blood flow but normal glucose utilization rates in hypertrophied areas that failed to increase during physical exercise, suggesting the presence of myocardial ischemia (20). Camici et al. found an impaired global flow reserve not only in hypertrophied but also in nonhypertrophied areas (21) and concluded that the observed results were due to primary changes rather than regional hypertrophy. Their results are further supported by studies done by Choudhury et al. and Radvan et al., who observed greater impairment of coronary vasodilatory reserve in patients with hypertrophic cardiomyopathy than in patients with secondary hypertrophy (22) and also observed no alterations in athletes with physiological myocardial hypertrophy (23). Recently, a prognostic value for PET-determined impaired flow reserve has been demonstrated in a long-term study with follow-up from 6 to 10 years (24).
especially pronounced in the septum. In patients symptomatic for chest pain, Nienaber et al. observed reduced blood flow but normal glucose utilization rates in hypertrophied areas that failed to increase during physical exercise, suggesting the presence of myocardial ischemia (20). Camici et al. found an impaired global flow reserve not only in hypertrophied but also in nonhypertrophied areas (21) and concluded that the observed results were due to primary changes rather than regional hypertrophy. Their results are further supported by studies done by Choudhury et al. and Radvan et al., who observed greater impairment of coronary vasodilatory reserve in patients with hypertrophic cardiomyopathy than in patients with secondary hypertrophy (22) and also observed no alterations in athletes with physiological myocardial hypertrophy (23). Recently, a prognostic value for PET-determined impaired flow reserve has been demonstrated in a long-term study with follow-up from 6 to 10 years (24).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree