PET-CT and SPECT-CT in Non-Neoplastic, Noninflammatory Pediatric Diseases
Liselotte Højgaard
Lise Borgwardt
With the recent availability of combined single-photon emission computed tomography (SPECT) and computed tomography (CT), along with the advent of high-performance CT, it is anticipated that this modality will be used for imaging benign pediatric diseases, for example, in lung scintigraphy for the imaging of congenital malformation and cystic fibrosis and for the workup for lung transplantations. In Kawasaki disease and complicating aneurisms of the coronary arteries, a “one-stop shop” 64-slice CT combined with cardiac SPECT might be useful. SPECT-CT will probably be useful in Meckel’s diverticulum scintigraphy and perhaps also in cholescintigraphy of the newborn with jaundice. In benign pediatric diseases of the bones, recent reports are available on the beneficial use of SPECT-CT, especially for the spine. In the brain, positron emission tomography (PET)-CT and SPECT-CT are useful for the diagnosis of epilepsy but also in rarer diseases such as Sturge-Weber syndrome, Rett syndrome, neurofibromatosis, tuberous sclerosis, sickle cell encephalopathy, and traumatic brain injury. PET has been evaluated, and PET-CT might be play a role in pediatric imaging in the future.
Introduction
Combined single-photon emission computed tomography (SPECT)–computed tomography (CT) cameras have been available with low-dose CT for a number of years, and recently new scanners with high-performance CT with up to 64 slices have been introduced. Combined positron emission tomography (PET)-CT scanners have been commercially available since 2001. In children, SPECT-CT with low-dose CT has been used increasingly for iodine 123 (123I)-MIBG (meta-iodobenzylguanidine) scintigraphy for neuroblastoma, for indications for octreotide scintigraphy, and for indium 111 (111In) white blood cell (WBC) scintigraphy for infections. In the case of non-tumor and non-infection indications, the availability of SPECT-CT with multi-slice CT will probably lead to more widespread use of this modality for a variety of indications in pediatric nuclear medicine.
SPECT-CT and PET-CT in Benign Pediatric Diseases of the Brain
In pediatric non-neoplastic neurology, PET and SPECT are regarded as accurate and noninvasive modalities for studying brain activity, elucidating the complexities of the
developing brain, and understanding disease processes (1). The maturational changes relevant for pediatric neurological interpretation were described by Chugani et al. (2) using PET (Fig. 66.6). Muller et al. (3) studied brain organization for language in children, adolescents, and adults with left hemispheric lesions. They found enhanced postlesional plasticity in childhood.
developing brain, and understanding disease processes (1). The maturational changes relevant for pediatric neurological interpretation were described by Chugani et al. (2) using PET (Fig. 66.6). Muller et al. (3) studied brain organization for language in children, adolescents, and adults with left hemispheric lesions. They found enhanced postlesional plasticity in childhood.
A simplified quantification method for measuring cerebral glucose utilization in early infancy was published by Suhonen-Polvi et al. (4). If blood samples cannot be obtained, SUV represents an alternative for estimation of cerebral glucose uptake and thus for interindividual comparison of patients.
The majority of publications discussing pediatric brain PET and SPECT in clinical neurology are concerned with the diagnosis of epilepsy (Fig. 68.1). PET and SPECT are used in combination with clinical findings, neurophysiologic evaluation, electroencephalography monitoring, and magnetic resonance imaging (MRI) studies, and they have proven very useful for exact preoperative localization of the epileptogenic focus. However, authors of comparative localization studies have come to divergent conclusions regarding the priority of the different methods. The International League against Epilepsy (ILAE) commission report recommends the priority of the different examinations (5).
Nearly all results of PET in children with epilepsy have been obtained using interictal studies because of the very complicated operational problems associated with ictal PET studies and the long period of tracer uptake in fluorodeoxyglucose (FDG) PET (see also Chapter 25). Nevertheless, some favorable results have been reported employing ictal PET in patients with continuous or frequent seizures (6).
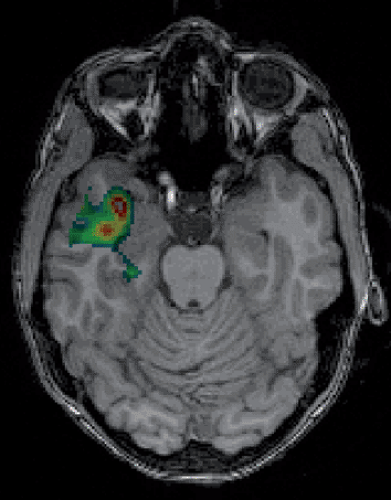
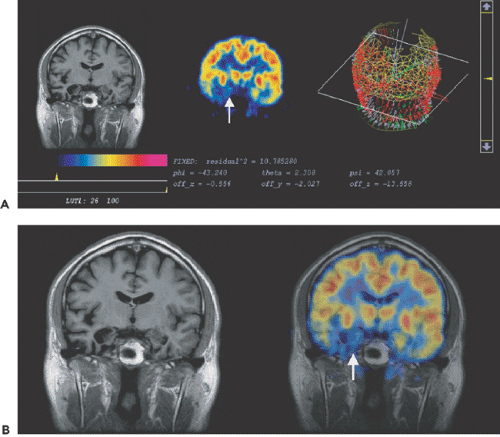 Figure 68.1 Epileptogenic focus due to hippocampal atrophy (arrows). (A: MR, PET and 3D image, B: MR and PET-MR fusion image). |
In addition to PET with FDG, other tracers, such as carbon 11 [11C]flumazenil (FMT), have been used in children with epilepsy (7). The authors of these studies concluded that PET with other tracers is significantly more sensitive than FDG-PET for the detection of cortical regions of seizure onset with frequent spiking in patients with extratemporal epilepsy, whereas PET with both FDG and [11C]flumazenil shows only limited sensitivity in the detection of cortical areas with rapid seizure spread. Alpha-11C-methyl-L-tryptophan (AMT) was found to be a promising diagnostic tool for localization of epileptogenic areas in patients with tuberous sclerosis (8).
Ictal–interictal SPECT maps cerebral perfusion during and shortly after single seizures, compared with the interictal state. Often in unilateral mesial temporal lobe epilepsy–hippocampus sclerosis (MTLE-HS), the retention of interictal technetium 99m (99mTc)-HMPAO (hexamethylpropyleneamine oxime) or 99mTc-ECD (ethyl cysteinate dimer) is mildly or moderately decreased in the anterior temporal lobe ipsilaterally, but false lateralization of interictal temporal lobe hypoperfusion occurs in perhaps 10% of patients. When the radioligand is injected during a complex partial seizure, the temporal lobe of ictal onset usually shows a great increase in radioligand
retention, and false lateralization is quite rare when using coregistered ictal versus interictal temporal lobe perfusion maps, the subtraction ictal SPECT coregistered to MRI (SISCOM) (Fig. 68.2).
retention, and false lateralization is quite rare when using coregistered ictal versus interictal temporal lobe perfusion maps, the subtraction ictal SPECT coregistered to MRI (SISCOM) (Fig. 68.2).
Peri-ictal SPECT studies of temporal lobe epilepsy have shown a characteristic evolution in the regional perfusion, as the region of ictal hyperperfusion declines to severe hypoperfusion for several minutes postictally, and then within 20 minutes perfusion increases back to a milder degree of interictal hypoperfusion. This phenomenon has been called “postictal switch” and can cause false lateralization when interictal scans are compared with early postictal scans that are mistakenly considered to represent an ictal scan. Ictal–interictal SPECT studies require considerable expertise in the timing of the radioligand injection, which must be performed with continuous EEG. Thus it appears likely that ictal SPECT maps are areas of ictal onset and of most intense seizure propagation.
FDG-PET studies in term newborns with hypoxic–ischemic encephalopathy have shown that during the subacute period after perinatal asphyxia, cerebral glucose metabolism correlates well with the severity of encephalopathy and short-term clinical outcome (9,10). SPECT studies have also been used in studying perinatal asphyxia (11).
A summary of functional imaging of neuropsychiatric disorders in childhood was published by O’Tuama et al. (12). The focus has primarily been on attention deficit hyperactivity disorder (13,14), anorexia nervosa (15), bulimia nervosa (16), and obsessive–compulsive disorder (17).
FDG-PET has also been used to investigate different types of inflammatory neurological diseases in infants, such as Rasmussen encephalitis (18,19) (Fig. 25.6), and to study HIV-1–infected children born to seropositive mothers (20).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree