PET-CT and SPECT-CT of Bone Metastases
Klaus Strobel
Daniela B. Husarik
Thomas F. Hany
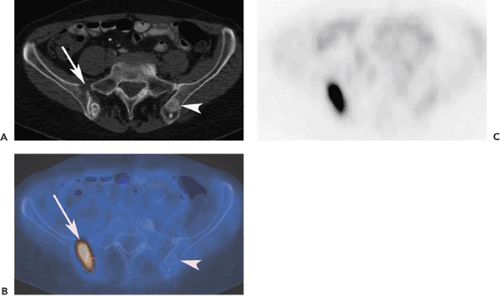
The skeletal system is the third most common localization of distant metastases of malignant tumors, following the lungs and liver. Early detection of bone metastases (BM) allows for accurate staging, rapid initiation of therapy, and possible reduction of related morbidity. Diagnostic imaging plays an important role in the detection of distant metastases and especially of bone metastases (BM). There is a wide range of imaging methods to assess bone involvement: conventional x-ray studies, computed tomography (CT), magnetic resonance imaging (MRI), and several nuclear medicine techniques including bone scintigraphy (BS) with single-photon emission computed tomography (SPECT) or SPECT-CT, and positron emission tomography (PET)-CT, using different tracers, including the PET tracer fluorodeoxyglucose (FDG),18F-fluoride, 18F-choline (FCH), and 18F-DOPA (dihydroxyphenylalanine). BS with technetium-labeled phosphate complexes has been the “work horse” since the early 1980s in detecting bone metastases with high sensitivity. Introduction of SPECT and SPECT-CT imaging techniques have increased specificity in the differentiation of bone metastases as an adjunct to planar bone scintigraphy. Additionally, lesions detected in BS can be correlated with conventional x-ray views or CT to asses the stability and search for pathologic fractures. FDG-PET has shown a comparable sensitivity for the detection of BM compared with planar BS. But FDG-PET has some limitations, depending on the type of malignancy: it is inferior to BS in detecting osteoblastic metastases (breast or prostate) and BM in patients with osteosarcoma. FDG-PET/CT can overcome some of these limitations if the CT information is not used only for anatomic localization of PET lesions (see Fig. 52.1). 18F-fluoride is superior to FDG-PET in FDG-negative tumors. FDG-PET seems to be superior in early stages of bone metastases. Initial results indicate that FCH is a potential substance for N and M staging of patients with prostate cancer with increased PSA levels.
General Remarks On Imaging
At an early stage, x-ray–based techniques may fail to detect bone metastases (BM), since frequently no morphologic alterations are present. Morphologic imaging modalities can be successful in the detection of these lesions only if bony structures (and not only soft tissue) have been altered by the malignant process. Bone scanning (BS) with Tc-phosphonate compounds has been established as the gold standard for the detection of bone metastases in the workup of several malignant diseases such as breast or prostate cancer. It represents a molecular rather than an anatomic imaging technique, as it measures the molecular process of bone accretion. This technique is very sensitive in detecting skeletal metastases; however, because of increased tracer uptake in degenerative, inflammatory, and other benign diseases, it has relatively low specificity. BS in combination with SPECT, but particularly SPECT-CT, can decrease the need to perform additional investigations, because the combination of BS and x-ray imaging techniques in combination are sensitive and specific. The value of BS has its limitations in detecting BM as in some tumors such as renal cell carcinoma, and multiple myeloma tracer accumulation occurs in only 20% to 50% of
lesions. In addition, the “flare effect” is a well-known phenomenon, in which successful therapy results in a benign reparative process in the affected bone, thus also showing increased activity on BS. This precludes the use of BS to monitor treatment response of BM (1,2).
lesions. In addition, the “flare effect” is a well-known phenomenon, in which successful therapy results in a benign reparative process in the affected bone, thus also showing increased activity on BS. This precludes the use of BS to monitor treatment response of BM (1,2).
MRI is an imaging modality with high diagnostic performance in the detection of bone metastases, permitting the evaluation of bone marrow, spinal cord, and soft tissue structures. In the spine and pelvis, MRI is more sensitive than planar BS, whereas BS is more sensitive in detecting BM in the ribs and skull (3). With further technologic development, whole-body MRI may become widely used for oncologic staging of bone metastases not only in pediatric patients but also in adults. With the use of rapid sequences (diffusion-weighted echo-planar MRI, STIR-sequences) and new scanner designs, whole-body images can be obtained in approximately 15 minutes. The main advantage of MRI is the absence of radiation exposure. However, initial studies comparing whole-body MRI to FDG-PET have demonstrated that PET imaging in the detection of bone metastases is superior to MRI (4).
In the daily routine, most PET and PET/CT studies are performed using FDG as tracer. FDG, like MRI, seems to be highly sensitive in detecting BM at early stages when only the bone marrow itself is affected. Osteolytic BM have a higher glycolytic rate compared with relatively acellular osteoblastic BM; therefore, FDG-PET may be more successful in detecting osteolytic than osteoblastic metastases (5) (Fig. 52.1). Therefore, the architecture and morphology of metastases (sclerotic, mixed, lytic) is of major importance. The anatomic localization is relevant in this respect for detection in PET imaging, because small lesions in the long bones frequently have an osteoblastic response and are well detected with BS or with 18F-fluoride PET. Inversely, vertebral body lesions often show limited osteoblastic response and may be better detected with FDG-PET.
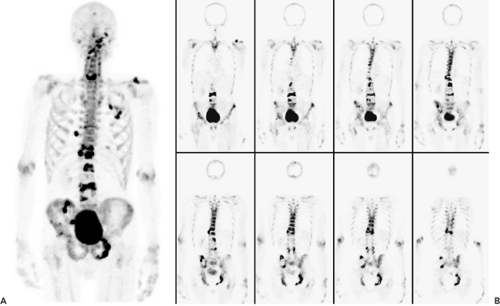
18F-fluoride is an interesting nonspecific bone-seeking PET tracer with an accumulation mechanism similar to the Tc-phosphonate compounds used in conventional bone scanning. Both depend on bone reparation rather than tumor cell viability (Fig. 52.2). The first pass blood extraction rate of 18F-fluoride through the capillary membrane is almost 100% in comparison with only 64% of the larger 99mTc-diphosphonate complexes (6). Higher spatial resolution and shorter acquisition times of 18F-fluoride PET imaging compared with conventional bone scanning results in better overall image quality (7). Initial studies indicate that 18F-fluoride is more sensitive than conventional bone scanning in detecting skeletal metastases in patients with prostate, lung, and thyroid cancer (8). Most additional lesions noted were located in the spine. In a study by Even-Sapir et al. (9), 18F-fluoride–PET was compared with 18F-fluoride–PET/CT in the assessment of malignant skeletal disease in 44 patients. In a patient-based analysis, the sensitivity of 18F-PET and 18F-PET/CT was 88% and 100%, respectively (p < 0.05) and the specificity was 56% and 88%, respectively. Because there are data that suggest that 18F-fluoride–PET may be cost effective, some authors expect that “classic” BS will be replaced by 18F-fluoride–PET completely in the coming years (7,10). Some authors have proposed a two-in-one PET investigation with combined application of FDG and 18F-fluoride for complete staging of skeletal and soft tissue metastases. Whether this approach will play a role in the future has to be evaluated in further studies (11).
Bone Metastases in Breast Cancer
Bone is the most common site of breast cancer metastases (see also Chapter 45). Bone is also the most frequent localization of recurrence after treatment. Up to 90% of patients who have terminal breast cancer develop bone metastases (12). Although prognosis of women with visceral metastases from breast cancer is poor, bone metastases in breast cancer does not necessarily imply a poor outcome. Early detection and treatment of bone metastases prior to the development of functional deficits is important. In most institutions, bone scanning is the method of choice to detect bone metastases in breast cancer patients. Bone scanning is widely available, a whole-body method, and very sensitive. A negative bone scan has a high negative-predictive value to exclude bone metastases and can be used as a baseline investigation. Because increased bone uptake is not specific for tumor, further evaluation of active regions with x-ray views, SPECT, SPECT/CT, or MRI is sometimes necessary for correct diagnosis. There is a controversial discussion in the literature concerning whether BS or FDG-PET is more effective in detecting bone metastases in breast cancer (Fig. 52.3). In a recently published paper by Uematsu et al. (13), SPECT was superior to FDG-PET in detecting bone metastases, and the authors concluded that surveillance of metastatic spread to the skeleton in breast cancer patients based on FDG-PET alone is inadequate. Breast cancer metastases are often osteoblastic, and several publications point out that, because of the lower metabolic activity of osteoblastic compared with osteolytic metastases, the former may elude detection with PET imaging alone (14). Interestingly, in this study the patients with a mixed pattern or sclerotic metastases had a significantly better survival than patients with osteolytic lesions. Osteolytic lesions have a higher glycolytic rate, grow rapidly, and are aggressive. In contrast, osteoblastic metastases are relatively acellular. Cook et al. (5) measured the mean SUV of sclerotic, mixed, and osteolytic metastases to be 0.95, 3.6, and 6.6, respectively, in breast cancer patients. Nakai et al. (15) reported that the visualization rate of osteoblastic bone metastases with bone scintigraphy/FDG-PET was 100%/55.6%, 70.0%/100.0% for the lytic type and 84.2%/94.7% for the mixed type in 89 breast cancer patients. Overall there was no significant difference of FDG-PET compared with bone scintigraphy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree