PET-CT and SPECT-CT of Malignant Melanoma
Hans C. Steinert
In our institution, whole-body integrated positron emission tomography (PET) and computed tomography (CT) with fluorodeoxyglucose (FDG) is restricted to patients with high-risk malignant melanoma (Breslow tumor thickness >4 mm or known metastasis). The preselection of patients ensures high effectiveness of PET-CT imaging. This chapter discusses the clinical background, the staging and classification systems, prognostic factors, and various staging methods to understand the high value and effectiveness and the best indications of PET and integrated PET-CT scanning in malignant melanoma. It is well known that FDG is not specific for tumors. To avoid false-positive FDG-PET results, typical nonmalignant “hot spots” that mimic solitary or generalized metastases of malignant melanoma are discussed. Well-known limitations of FDG-PET such as microscopic tumor spread to regional lymph nodes or brain metastases are discussed as well. In addition, results of the additional incremental value of integrated PET-CT are presented. Early detection of lung metastases of melanoma by FDG-PET alone is limited. In an ongoing prospective study, we have found that dedicated interpretation of the CT part of integrated PET-CT improves the early detection of pulmonary metastases of melanoma.
Introduction
In 2004, malignant melanoma was the fifth and seventh most common cancer in men and women, respectively, in the United States. The incidence of cutaneous malignant melanoma among whites living in the United States, Australia, and in Western Europe is still increasing, but this increase is partly owing to improved screening programs. According to the American Cancer Society, malignant melanoma accounts for 1% to 2% deaths per year.
In 2002, the American Joint Committee on Cancer introduced a revised staging system. Malignant melanoma prognostic factors now include primary tumor thickness, microscopic or macroscopic lymph node metastasis, number and location of lymph node metastases, and the sites of distant metastases. Stages I and II refer to localized melanoma. Early malignant melanoma is curable by means of surgical excision. Stage III melanoma refers to cases with lymph node metastases, either in regional nodes or as satellite or in-transit metastases. Patients with distant metastases are classified as stage IV. Despite the enormous progress of modern oncology, the prognosis of metastasizing melanoma has remained particularly poor. Patients with regional lymphatic metastasis have a cure rate of approximately 20%, whereas no curative treatment is currently available for generalized metastatic melanoma.
Accurate staging of malignant melanoma is essential for the early detection of metastases and for choosing the appropriate treatment. The high mortality rate of malignant melanoma is owing to its early hematogenous spread, and the location of metastases is unpredictable. The skin, subcutaneous tissue, and distant lymph nodes are the most
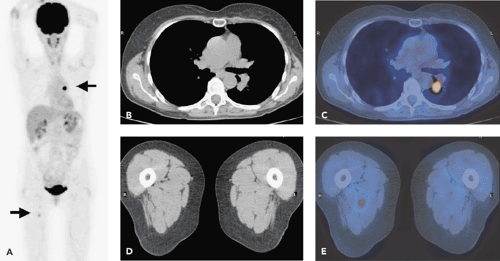
common sites of distant metastases, but melanoma can metastasize to all organs. The early detection and surgical excision of single distant metastases are important for improving survival (Fig. 54.1).
common sites of distant metastases, but melanoma can metastasize to all organs. The early detection and surgical excision of single distant metastases are important for improving survival (Fig. 54.1).
Whole-body fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging has been shown to be superior to conventional imaging methods in staging patients with high-risk melanoma. In many institutions worldwide, FDG-PET imaging has nearly replaced the standard battery of imaging tests for staging high-risk malignant melanoma, such as chest x-ray films, ultrasound of lymph node stations and abdomen, computed tomography (CT) of the chest and abdomen, and bone scan. With these conventional techniques, false-negative and false-positive findings are quite common. Whole-body FDG-PET has a major impact in the management of patients with melanoma. Surgical resection is the treatment of choice for regional lymph node metastases or single distant metastases. Whole-body FDG-PET should be used to exclude occult metastases in patients in whom surgery is planned. If multiple metastases are present, chemotherapy is the therapy of choice. In extended disease, only palliative therapy is indicated. Whole-body FDG-PET plays an important role in the evaluation of patients, when immunotherapy is considered. Adjuvant treatment with recombinant interferon alpha is indicated only in disease-free patients after resection of high-risk melanoma. FDG-PET is also useful in evaluating the treatment response.
However, limitations of FDG-PET imaging have been recognized. The sensitivity of FDG-PET in the detection of microscopic tumor spread to the sentinel lymph node is poor. Although malignant melanoma is one of the most avidly FDG-accumulating tumors, the spatial resolution of 5 mm of dedicated PET scanners is limited. The early spread of disease to regional lymph nodes often involves only some tumor cells. It has been shown that FDG accumulation in nodal metastasis is dependent on the size of the metastasis and on nodal tumor involvement of more than 50% or capsular infiltration. Sentinel lymph node (SLN) scintigraphy and biopsy have been established as accurate baseline techniques for the determination of tumor spread to regional lymph nodes. Because of physiologic FDG uptake in the brain, FDG-PET is of limited value to screen for brain metastases. In our institution, however, we always include the brain in the whole-body PET examination. In some cases, unknown and unexpected brain metastasis can be detected. In the assessment of pulmonary metastases of melanoma, FDG-PET shows a higher specificity but lower sensitivity than CT. In our experience, this limitation can be overcome by the use of PET-CT imaging. Growing pulmonary nodules even without FDG accumulation are highly suspicious for metastases.
In our institution, whole-body integrated PET-CT scanning with FDG has become the standard diagnostic tool for staging patients with high-risk malignant melanoma,
that is, Breslow thickness greater than 4 mm or known metastases. For baseline staging, SLN scintigraphy and biopsy are routinely performed in all patients with a malignant melanoma.
that is, Breslow thickness greater than 4 mm or known metastases. For baseline staging, SLN scintigraphy and biopsy are routinely performed in all patients with a malignant melanoma.
Clinical Characteristics
Cutaneous malignant melanoma is the most aggressive cancer of the skin. No other tumor is increasing faster in number of new cases diagnosed (1). As with most cancers, the causes of malignant melanoma are multifactorial. Numerous studies have demonstrated that the development and progression of melanoma are based on increasing levels of cutaneous solar exposure, especially ultraviolet B radiation, in combination with the genotype, phenotype, and immunocompetence of the patient.
Melanomas can be located anywhere in the body, but most commonly occur on the lower extremities in women and on the back in men. Any pigmented lesion with a change in size, configuration, or color should be considered a potential melanoma, and an excisional biopsy should be performed. Fortunately, nowadays most patients are diagnosed early, so that malignant melanoma can be cured with surgical excision of the primary lesion. Nevertheless, late diagnosis in locations that are not visible to the patient, such as the scalp, neck, and back, or in a plantar location are fairly common. The widely varying mortality reports in the literature depend more on the stage of diagnosis than on variations in surgical and treatment technique.
Classification and Staging
Histologic verification and accurate microstaging of tumor thickness are essential for treatment decisions and to predict the risk of metastases. Two methods have been used. The Breslow microstaging method measures the thickness of the lesion using an ocular micrometer to define the total vertical height of the melanoma from its surface to the deepest part of the lesion. The Clark microstaging method categorizes different levels of invasion that reflect depth of penetration into the dermal layers and the subcutaneous fat (i.e., levels II, III, IV, or V). It has been demonstrated that the Breslow tumor thickness is the most important prognostic factor in clinically localized melanoma and is a more reproducible parameter than interpreting the level of invasion (2).
In 2002, the American Joint Committee on Cancer introduced a revised staging system for malignant melanoma (3). Stages I and II include localized melanomas up to 2.0 mm thickness and negative lymph nodes. Stage III includes regional lymph-node metastases. Stage IV describes cases with distant metastases. Important prognostic factors such as microscopic or macroscopic nodal involvement, the number of positive nodes, the anatomic location of nodal and distant metastases have been included in this staging system.
Lesions with a Breslow thickness less than 1.0 mm have an excellent prognosis not differing significantly from that of the general population, whereas those greater than 4 mm in thickness have a 10-year survival of less than 40%. Once patients develop metastases, other prognostic factors have to be considered. The number of metastatic nodes has a significant prognostic value. The high mortality of patients with melanoma is caused by its early hematogenous spread. The skin, subcutaneous tissue, and distant lymph nodes are the most common sites of distant metastases, but melanoma can metastasize to all organs. Early detection and surgical excision of single distant metastases are important in improving the prognosis. Significant factors predicting survival in patients with distant metastases are the number of metastatic sites and the remission duration (less than 12 months versus more than 12 months). The results of vaccine therapies for melanoma suggest that immune and clinical responses are promising in patients with metastatic disease (4,5,6).
Our group has demonstrated that whole-body staging at baseline remains limited (7). One hundred consecutive patients with malignant melanoma and a tumor thickness greater than 1.0 mm were enrolled in the study. All patients underwent extensive baseline staging including physical examination, ultrasound (US) of the abdomen and regional lymph nodes, SLN scintigraphy and biopsy, chest x-ray film, and whole-body FDG-PET. Twenty-six percent of patients had a positive sentinel lymph node among 90% with microscopic disease. The macroscopic nodal metastases could be detected by physical examination, US, or PET.
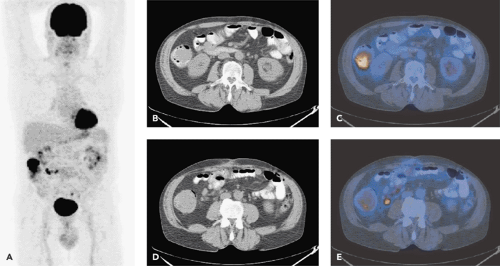
Patients with a melanoma thickness of 1.0 to 4.0 mm have an increased risk of occult regional nodal metastases, but have a relatively low risk (less than 20%) of distant metastases. Patients with melanomas thicker than 4.0 mm have a high risk (greater than 70%) of distant metastases. In our institution, an integrated PET-CT with FDG will be performed for whole-body staging only in patients with a primary malignant melanoma greater than 4.0 mm or known metastasis. Because of the erratic pattern of distant metastases, whole-body staging is recommended (Fig. 54.2). The clinical course of melanoma can be characterized by the risk of relapse and death well beyond 10 years after the initial diagnosis. Therefore, lifetime annual clinical follow-up has been recommended. According to the Guidelines of the Swiss Society of Dermatology, PET or PET-CT scanning is annually recommended in the first 5 years after the diagnosis of high-risk melanoma.
Staging Methods
In the past, a combination of conventional imaging modalities was used for staging of malignant melanoma, that is, chest x-ray film; ultrasound of the abdomen and lymph nodes of the axilla, cervical region, and groin; and CT. However, these methods are intrinsically spot imaging methods and better used to evaluate a given region rather than the entire body. Furthermore, identification of tumor tissue
(e.g., in normal-sized lymph nodes) is difficult with these methods. If a lesion is detected, further procedures such as biopsies or follow-up examinations are necessary to confirm or exclude malignancy. CT is known to have a high rate of false-positive findings if applied as a screening method in patients with malignant melanoma (8). Because of the limitations of morphologic imaging modalities, several radiopharmaceuticals have been used in nuclear medicine to visualize metastases of melanoma. These include 67gallium-citrate (9), immunoscintigraphy with monoclonal antibodies (10), 111indium-pentetreotide (11), 99mtechnetium-MIBI (12), 123iodine-methyltyrosin (13), 18fluorine-fluoroethyltyrosine (14), 123iodine-iodobenzufuran (15), 18fluorine-fluorodopa (16,17), 76bromine-bromodeoxyuridine (18), and 18fluorine-fluorodesoxyuridine (19). Because of limited sensitivity, these radiotracers were found to be suitable for screening of melanoma only in exceptional cases. Several 11carbon-labeled radiopharmaceuticals have been used for experimental studies in malignant melanoma. Because of the short half-life of 11carbon, the clinical use of these tracers for whole-body staging remains limited.
(e.g., in normal-sized lymph nodes) is difficult with these methods. If a lesion is detected, further procedures such as biopsies or follow-up examinations are necessary to confirm or exclude malignancy. CT is known to have a high rate of false-positive findings if applied as a screening method in patients with malignant melanoma (8). Because of the limitations of morphologic imaging modalities, several radiopharmaceuticals have been used in nuclear medicine to visualize metastases of melanoma. These include 67gallium-citrate (9), immunoscintigraphy with monoclonal antibodies (10), 111indium-pentetreotide (11), 99mtechnetium-MIBI (12), 123iodine-methyltyrosin (13), 18fluorine-fluoroethyltyrosine (14), 123iodine-iodobenzufuran (15), 18fluorine-fluorodopa (16,17), 76bromine-bromodeoxyuridine (18), and 18fluorine-fluorodesoxyuridine (19). Because of limited sensitivity, these radiotracers were found to be suitable for screening of melanoma only in exceptional cases. Several 11carbon-labeled radiopharmaceuticals have been used for experimental studies in malignant melanoma. Because of the short half-life of 11carbon, the clinical use of these tracers for whole-body staging remains limited.
Whole-body PET and PET-CT Imaging with FDG
Malignant melanoma shows one of the highest FDG uptakes of all tumors (20). Whole-body PET using FDG has been proven to be a highly effective and cost-saving modality to screen for metastases of malignant melanoma throughout the body. With the exception of the brain and the lung, whole-body FDG-PET can largely replace the standard battery of imaging tests currently performed on high-risk patients (Figs. 54.2 and 54.3).
Our group reported in a study of 33 patients a sensitivity of 92% with a specificity of 77% for reading the PET images without clinical information (21). Specificity improved to 100% when clinical information such as location of biopsy sites or location of subcutaneous injections of interferon were obtained. PET was also highly accurate in differentiating benign from malignant lesions. In six patients (18%), whole-body PET depicted previously unknown metastases. In four of these six patients, the metastases were surgically removed. The excellent results in staging high-risk melanoma patients with whole-body FDG-PET were confirmed in larger patient studies in other PET centers worldwide (22,23,24,25,26,27,28).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree