This review article summarizes the clinical applications of established and emerging PET tracers in the evaluation of the 5 most common gynecologic malignancies: endometrial, ovarian, cervical, vaginal, and vulvar cancers. Emphasis is given to 2-deoxy-2-[ 18 F]fluoro- d -glucose as the most widely used and studied tracer, with additional clinical tracers also explored. The common imaging protocols are discussed, including standard dose ranges and uptake times, established roles, as well as the challenges and future directions of these imaging techniques. The key points are emphasized with images from selected cases.
Key points
- •
2-Deoxy-2-[ 18 F]fluoro- d -glucose (FDG) PET generally has high sensitivity for advanced disease but limited specificity, making it a useful tool for late-stage disease and detecting malignant recurrence, but limiting its role for diagnosis.
- •
FDG uptake has been correlated with tumor aggressiveness, and can help with treatment planning, particularly of radiation treatment fields.
- •
Vaginal and vulvar cancers are less common than endometrial, ovarian, and cervical cancers, and investigations on the role of PET are less conclusive and require further studies.
- •
Many new and emerging PET tracers are promising for evaluating and mapping different receptors, cell proliferation rates, and even hypoxia, helping to direct treatment, but most still require additional testing before they are ready for standard clinical use.
Introduction
Approximately 94,000 new cases of gynecologic cancer are diagnosed in the United States each year. Gynecologic cancers are typically separated into 5 groups based on anatomy, listed here from most to least common: endometrium/uterine body, ovary, cervix, vagina, and vulva. Incidence rates are summarized in Table 1 . These cancers have diverse clinical presentations and prognoses, and their treatment typically includes a combination of radical surgery, chemotherapy, and/or radiotherapy based on the stage of disease.
| Cancer Location | Incident Cases per 100,000 |
|---|---|
| Uterine body | 26.82 |
| Ovary | 11.18 |
| Cervix | 7.60 |
| Vulvar | 2.62 |
| Vaginal | 0.66 |
The stage of disease at diagnosis is predictive of prognosis and is crucial for selection of the best mode of therapy. Gynecologic cancers are typically staged using the International Federation of Gynecology and Obstetrics (FIGO) criteria. Imaging plays an important role in pretreatment evaluation of patients with gynecologic malignancies as well as in the follow-up for response assessment to therapy and detection of disease recurrence. Imaging of the female pelvis can be achieved using a combination of ultrasonography (US), computed tomography (CT), magnetic resonance (MR) imaging, and PET/CT using 2-deoxy-2-[ 18 F]fluoro- d -glucose (FDG) and other emerging radiopharmaceuticals. PET/CT is known to be more accurate than CT or MR imaging alone for staging and can help direct management.
This article discusses the use of FDG-PET/CT and PET/MR for clinical evaluation of the most common types of gynecologic cancers: endometrial, ovarian, cervical, vaginal, and vulvar cancers.
PET radiopharmaceuticals and imaging protocols
Anatomic evaluation of gynecologic malignancies is typically performed with a combination of CT and MR imaging. PET is commonly coupled with concurrent CT for attenuation correction and anatomic localization, which is often of lower resolution than dedicated CT studies and is often performed without contrast. PET/MR imaging is a more recent innovation, but access remains limited to larger institutions and it is not in widespread use. To allow a more in-depth PET review, anatomic evaluation is not the focus of this article. The focus is on FDG because of its widespread clinical use and accepted role in evaluation in malignant diseases. However, it also briefly reviews clinically approved non-FDG radiopharmaceuticals, including somatostatin-receptor and steroid-receptor imaging agents, as well as radiopharmaceuticals that are currently used in research settings that have clinical potential, including human epidermal growth factor receptors (EGFR), DNA-precursor use, and cell hypoxia imaging tracers. The PET tracers discussed here are summarized in Table 2 along with key procedural parameters.
| PET Tracer | Half-Life (T 1/2 ) | Typical Dose | Uptake Time | Fasting State |
|---|---|---|---|---|
| 18 F-FDG | 109.7 min | 370–740 MBq (10–20 mCi) | 60 min | At least 4 h |
| 68 Ga-DOTATOC 68 Ga-DOTATATE 68 Ga-DOTANOC | 68 min | 132–222 MBq (4–6 mCi); should not be <100 MBq (2.7 mCi) | 60 min | Not required |
| 64 Cu-DOTATATE | 12.7 h | 148 MBq (4 mCi) | 45–90 min | Not required |
| 18 F-FES | 109.7 min | 222 MBq (6 mCi); range 111–222 MBq (3–6 mCi) | 60 min; range 20–80 min | Not required |
| 89 Zr-labeled lumretuzumab | 3.27 d | 37 MBq (1 mCi) | 2, 4, and 7 d | Not required |
| 18 F-FLT | 109.7 min | 2.6 MBq/kg (0.07 mCi/kg); maximum dose 185 MBq (5 mCi) | 60–70 min | Not required |
| 18 F-FMISO | 109.7 min | 3.7 MBq/kg (0.1 mCi/kg); maximum 260 MBq (7 mCi) | ≥2 h | Not required |
| 60 Cu-ATSM | 23.7 min | 481–740 MBq (13–20 mCi) | 60-min dynamic imaging and/or static imaging at 30 min | Not required |
| 64 Cu-ATSM | 12.7 h | 925 MBq (25 mCi) |
Glucose Metabolism
FDG ( 18 F: half-life T 1/2 = 109.7 minutes), a glucose analogue, is the most common tracer used for clinical evaluation of patients with gynecologic malignancies. The biological basis for the use of FDG in oncology is the Warburg effect, which describes an increase in glycolysis under aerobic conditions and is characteristic of the malignant state. FDG is taken up by the cell using glucose transporters and phosphorylated by hexokinase to FDG–6 phosphate (FDG-6P). FDG-6P is not a good substrate for further metabolism and is trapped within the cell, because glucose 6-phosphatase is markedly downregulated in cancer cells.
Patient preparation for FDG-PET imaging of gynecologic tumors typically follows the Society of Nuclear Medicine and Molecular Imaging guidelines, which includes fasting for at least 4 hours and fasting blood glucose level less than or equal to 200 mg/dL. Typical doses are within 10 to 20 mCi (370–740 MBq). Urinary tract preparation that involves placement of a Foley catheter, intravenous administration of fluids, and furosemide may be performed for evaluation of gynecologic cancers for evaluating lesions close to the bladder. Imaging typically begins 60 minutes after administration of FDG. Standard imaging from the skull base to the thighs typically takes approximately 20 minutes, but depends on patient size and scanner technology. Anatomic imaging protocols differ between institutions and scanners. Although CT oral contrast is used in many centers, the use of intravenous contrast is controversial and limited to some centers. CT images are typically acquired before PET acquisition.
For PET/MR imaging, routine pelvic MR protocols are acquired with and without intravenous contrast, use of antiperistaltic medications, and intravaginal contrast. Acquired sequences differ between institutions, but typically include T1-weighted and T2-weighted sequences, diffusion-weighted imaging, and postcontrast imaging, which enables better evaluation of structures and possible tumor invasion. The use of dynamic contrast-enhanced imaging allows the evaluation of tissue perfusion and oxygenation. , Although PET/CT enables direct calculation of attenuation correction from the CT data, MR imaging relies on determining tumor composition and associated look-up tables. A common technique relies on Dixon sequences to delineate up to 4 materials within a given pixel, typically background/air, soft tissue, fat, and lung. Limitations include the inability to always properly delineate organs, particularly the lungs, and the inability to accurately identify cortical bone because of insufficient signal, both of which affect the attenuation correction and resulting standardized uptake value (SUV) accuracy. ,
PET analysis is both qualitative and quantitative. Qualitative analysis compares potential malignant uptake with physiologic uptake, including hepatic uptake, blood pool activity, and adjacent organ parenchymal activity. Quantitative uptake most commonly relies on the maximum SUV (SUV max ) because of ease of measurement, but SUV mean and peak, as well as metabolic tumor volume (MTV) and total lesion glycolysis (TLG), are becoming more accepted.
Estrogen Receptors
There are 2 types of estrogen receptors (ERs): ERα and ERβ. 16α- 18 F-fluoro-17β-estradiol (FES) is an estrogen analogue that was recently approved by US Food and Drug Administration (FDA) for imaging advanced breast cancer and provides imaging of ERs through selective binding of the ERα isoform. , However, its use in other cancers, including gynecologic cancers, is limited to research settings under investigational new drug applications. FES is the most investigated steroid-receptor tracer. Typical dose is 6 mCi (222 MBq) with a typical range of 3 to 6 mCi (111–222 MBq). Recommended imaging is 80 minutes (range of 20–80 minutes), but typically investigators use 60 minutes after administration before starting imaging. Fasting is not required.
Cell Proliferation
3′-Deoxy-3’-[ 18 F]fluorothymidine (FLT) , is a pyrimidine analogue of thymidine, a DNA precursor intended to evaluate cell proliferation rate, but is more a measure of S-phase fraction. Its uptake is via passive diffusion and facilitated transport by type 1 equilibrate nucleoside transporters (ENT1). Although FLT is a specific marker for cell proliferation and a better marker for evaluation of response to therapy than FDG, physiologic uptake of FLT in the bone marrow caused by increased cell proliferation, in the liver secondary to hepatic glucuronidation, and in the urinary tract as part of the renal clearance of the tracer represent the main limitations of the method. Investigators have used a dose of 0.07 mCi/kg (2.6 MBq/kg), maximum dose of 5 mCi (185 MBq), infused intravenously over 2 minutes, with PET images acquired 60 to 70 minutes after injection for imaging of cervical cancer. , Patient fasting was not an explicit requirement of the protocol.
Hypoxia
Tumor hypoxia inhibits radiation therapy by decreasing the availability of oxygen free radicals that cause tumor DNA damage and cell death. Tumor hypoxia also likely limits the efficacy of chemotherapy. Polarographic oxygen sensors are the gold standard of evaluating hypoxia but are limited by the invasive technique and inherent sampling limitations. PET offers a noninvasive means to reliably evaluate the entire tumor and multiple tumor sites at the same time. The PET agents for assessing hypoxia are in 2 groups. The first is fluorine-labeled nitroimidazoles such as 1-(2′nitro-1′-imidazolyl)-3-fluoro-2-propranol (FMISO) ; [ 18 F]fluoroazomycin arabinoside (FAZA), a second-generation 2-nitroimidazole; and [ 18 F]fluoroerythronitroimidazole (FETNIM), which is more hydrophilic than FMISO. The second group is copper-labeled diacetyl-bis( N 4-ethylthiosemicarbazone) (Cu-ATSM) analogues ( 60 Cu, T 1/2 = 23.7 minutes; 61 Cu, T 1/2 = 3.32 hours; 62 Cu, T 1/2 = 9.7 minutes; and 64 Cu, T 1/2 = 12.7 hours), which have neutral lipophilic molecules with high cell membrane permeability, and are reduced and trapped in hypoxic cells.
A standard dose of FMISO is 0.1 mCi/kg (3.7 MBq/kg) up to a maximum of 7 mCi (260 MBq). A combination of low tumor uptake, slow accumulation in hypoxic tissues, and slow clearance from normoxic tissue caused by the lipophilic nature of the tracer necessitates long wait periods, of 2 hours or more, between injection and imaging.
For 60 Cu-ATSM, a typical dose of 13 to 20 mCi (481–740 MBq) and for 64 Cu-ATSM, a typical dose of 25 mCi (925 MBq) injected intravenously, followed by 60 minutes of dynamic imaging , or static imaging at 30 minutes after injections, have been reported.
Endometrial cancer
Approximately 75% to 80% of patients with endometrial cancer are postmenopausal. This disease typically presents with abnormal bleeding resulting in early-stage diagnosis in 75% of patients. The risk factors include abdominal obesity, multiparity, late menopause, smoking, unopposed estrogen therapy, tamoxifen, Lynch syndrome, and diabetes; hormone replacement therapy, although a risk factor, is no longer typically prescribed. There are 2 histologic subtypes: type 1 are well differentiated estrogen-associated endometrioid adenocarcinomas accounting for 75% to 80% and expressing high levels of ERs. Type 2 are aggressive, undifferentiated, estrogen-independent cancers that typically develop in atrophic endometrium and include adenosquamous, serous papillary, clear cell, and undifferentiated types. FIGO staging of endometrial cancer, which was revised in 2009 and is summarized in Table 3 , does not include an imaging component. However, MR imaging is highly sensitive and specific for revealing important prognostic factors and thus, when available, is recommended as an adjunct to clinical examination. ,
| Stage I | Carcinoma confined to the uterus |
| IA | <50% invasion of the myometrium MR: abnormal signal intensity in endometrial cavity or confined to inner half of myometrium |
| IB | ≥50% invasion of the myometrium MR: extends into the outer half of myometrium |
| Stage II | Cervical stromal invasion without extension beyond the uterus MR: disruption or focal thinning of cervical stroma |
| Stage III | Carcinoma spread locally |
| IIIA | Serosal or adnexal invasion MR: disruption or irregular uterine contour caused by tumor; ovarian nodular tumor |
| IIIB | Vaginal or parametrial involvement MR: direct tumor extension of upper vagina or/and parametrial tissues |
| IIIC | Metastasis to pelvic or para-aortic lymph nodes |
| IIIC1 | Metastasis to pelvic lymph node MR: lymph nodes >8 mm in short axis |
| IIIC2 | Metastasis to para-aortic lymph node MR: lymph nodes >10 mm in short axis |
| Stage IV | Extension to the pelvic wall, lower one-third of the vagina, or hydronephrosis or nonfunctioning kidney |
| IVA | Bladder or bowel mucosal invasion MR: disruption of bladder or bowel muscular wall with mucosal invasion; not bullous edema |
| IVB | Distant metastases, including abdominal, or involvement of inguinal lymph nodes MR: tumor deposits at distal sites including peritoneal metastasis, bladder, bone liver metastasis, and distal lymph node metastases |
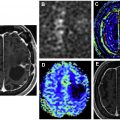
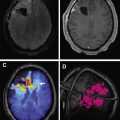
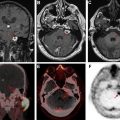
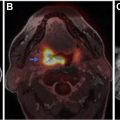
FIGO staging does include involvement of locoregional and distant nodal metastases, which PET can aid in detecting. Nodal metastatic pattern is predominately pelvic, following anterior pelvic, lateral pelvic, hypogastric, and presacral routes, but can then spread to para-aortic lymph nodes ; this reflects stage III disease, with para-aortic involvement being more advanced IIIC2 (see Table 3 ). Fig. 1 shows an example of recurrent endometrial cancer. More distant nodal spread to abdominal and/or inguinal lymph nodes reflects stage IV disease. Fig. 2 shows recurrent endometrial adenocarcinoma with distant metastasis.


FDG-PET
FDG-PET has a limited role in initial staging. Chang and colleagues reported a pooled sensitivity for the detection of pelvic and/or para-aortic metastasis of only 63% based on their meta-analysis, which is insufficient to replace lymphadenectomy. A more recent meta-analysis showed the overall pooled sensitivity, specificity, and area under the curve (AUC) of FDG-PET/CT for detection of lymph node metastases to be 72% (95% confidence interval [CI], 63%–80%), 94% (CI, 93%–96%), and 94% (CI, 85%–99%), respectively, with an overall diagnostic accuracy (Q∗ index) of 88%. Most patients with advanced disease also benefit from surgical debulking, and thus the results of FDG-PET are unlikely to deter surgery. However, FDG-PET can play a role in identification of distant metastases and treatment planning, particularly for radiation therapy. Furthermore, greater uterine tumor SUV max has been correlated with greater tumor aggressiveness. In particular, Kitajima and colleagues discovered that patients with SUV max 12.7 or greater had a significantly lower disease-free survival rate ( P = .00042). FDG-PET also has high sensitivity for detection of both local and distant recurrence, ranging from 85.7% to 100%, , and may therefore be useful for posttherapy surveillance and detection of recurrent disease. In a recent meta-analysis, FDG-PET/CT had an overall pooled sensitivity, specificity, and AUC for detection of endometrial cancer recurrence of 95% (CI, 91%–98%), 91% (CI, 86%–94%), and 97% (CI, 95%–98%), respectively, with overall diagnostic accuracy (Q∗ index) of 93%. The National Comprehensive Cancer Network (NCCN) suggests considering FDG-PET/CT if metastasis is suspected at initial staging and for evaluation of suspected recurrence.
2-Deoxy-2-[ 18 F]fluoro- d -glucose PET/Magnetic Resonance
Data on PET/MR are not as extensive as for PET/CT because of its recent clinical adoption. However, in gynecologic as well as nongynecologic malignancies, several investigators have already noted that PET/MR is superior to PET/CT in diagnosing brain and liver metastases, as well as removing the diagnostic uncertainty of some abdominal findings; nonetheless, PET/CT remains superior for diagnosing lung metastases. No statistically significant advantage of PET/MR compared with PET/CT has been ascertained, but data remain limited. Tsuyoshi and colleagues found that nonenhanced PET/MR has similar accuracy to contrast-enhanced CT and that greater SUV/apparent diffusion coefficient (ADC) ratio correlated with high-risk cancers. One of the important advantages of PET/MR, which contributed to the addition of MR, is the evaluation of myometrial invasion because of its high soft tissue resolution. Integrated PET/MR proved significantly more accurate than PET/CT. Bian and colleagues reported an overall detection accuracy of myometrial invasion for PET/CT and integrated PET/MR of 45.9% and 81.8%, respectively ( P <.001). The depth of myometrial invasion is an important prognostic factor because it strongly correlates with the risk of lymph node metastasis and prognosis in patients with endometrial cancer. Fig. 3 shows an example of FDG-PET/MR and the superiority of MR in evaluation of endometrial cancer.

16α- 18 F-fluoro-17β-estradiol PET
Endometrial cancer is traditionally divided into estrogen dependent (type 1) and estrogen independent (type 2). The presence of ERα and progesterone receptor in endometrial carcinoma correlates positively with clinical response rate and improved survival, and thus is a potential predictive and prognostic biomarker. Tsujikawa and colleagues , showed that increasing ratios of FDG/FES uptake can be used as a predictor of not only malignant versus benign tumors but also of malignant aggressiveness and stage. They determined an optimal cutoff ratio of 2.0 resulting in 73% sensitivity, 100% specificity, and 86% accuracy for differentiating malignant from benign lesions, outperforming the 77% accuracy for MR imaging, and noted that a cutoff ratio of 0.5 differentiated carcinoma from hyperplasia with 100% accuracy. , Results suggest that endometrial carcinoma has a reduced estrogen dependency and increased glucose metabolism. Zhao and colleagues evaluated FDG and FES as noninvasive biomarkers to assess uterine tumor hormone-receptor expression, glucose metabolism, and proliferation and as a tool to differentiate between uterine leiomyomas and sarcomas. They found a similar relationship of increased FDG/FES ratio in sarcomas compared with leiomyomas of 5.9 ± 3.9 versus 0.9 ± 0.5, respectively. Now that FES is approved in breast cancer, it is possible that this tracer will be available for evaluating gynecologic cancers in the future.
3′-Deoxy-3’-[ 18 F]fluorothymidine PET
Uterine leiomyoma is a common benign endometrial tumor, whereas leiomyosarcoma is a rare malignant tumor. However, leiomyomas occasionally resemble leiomyosarcoma on MR imaging and clinical presentation. Limited data are available for using FLT to distinguish between benign and malignant leiomyomas such as leiomyosarcomas. Yamane and colleagues showed that, although FDG and FLT both had sensitivities and negative predictive values (NPVs) for malignancies of 100%, FLT had better specificity, positive predictive value (PPV), and accuracy of 90.0%, 83.9%, and 93.3% compared with FDG values of 70.0%, 62.5%, and 80.0%, respectively. They also noted that FLT had better correlation with Ki-67 labeling index compared with FDG, with R 2 = 0.91 compared with R 2 = 0.26. Thus, it is possible that FLT-PET will become a valuable diagnostic tool for differentiating uterine leiomyosarcoma from leiomyoma in the future.
Ovarian cancer
Ovarian cancer is classified into 3 categories based on histology: epithelial, germ cell, and sex cord–stromal tumors. Epithelial ovarian cancer accounts for 95% of ovarian malignancies and originates from the surface epithelial layer of the ovaries or from the distal fallopian tubes. The ovaries are also a common location of metastatic disease, with 5% to 30% of ovarian cancers being metastatic, primarily from the gastrointestinal tract. Early diagnosis is difficult because of the lack of screening and nonspecific symptoms, leading to advanced stage (III or IV) at the time of diagnosis in most patients. Ovarian cancer is surgically staged and, thus, the FIGO staging system for this cancer, summarized in Table 4 , is surgically based and is defined by the extent and location of disease noted on cytoreduction (ie, debulking) surgery and biopsies.
| Stage I | Carcinoma limited to the ovary (or ovaries) or fallopian tubes without spread to nearby lymph nodes or to distant sites |
| IA | Carcinoma in 1 ovary or 1 fallopian tube, but not on their outer surfaces. No cancer cells in the ascites or washings from the abdomen and pelvis |
| IB | Carcinoma in both ovaries or fallopian tubes but not on their outer surfaces. No cancer cells in the ascites or washings from the abdomen and pelvis |
| IC | Carcinoma in both ovaries or fallopian tubes and any of the following are present: |
| IC1 | Surgical spill |
| IC2 | Capsule ruptured before surgery or tumor on ovarian or fallopian tube surface |
| IC3 | Cancer cells in the ascites or washings from the abdomen and pelvis |
| Stage II | Carcinoma in 1 or both ovaries or fallopian tubes with pelvic extension (below pelvic rim) or primary peritoneal cancer a without spread to nearby lymph nodes or to distant sites |
| IIA | Extension and/or implants on uterus and/or fallopian tubes and/or ovaries |
| IIB | Extension to other pelvic intraperitoneal tissues |
| Stage III | Carcinoma involves 1 or both ovaries or fallopian tubes, or primary peritoneal cancer, with cytologically or histologically confirmed spread to the peritoneum outside the pelvis and/or metastasis to the retroperitoneal lymph nodes without distant metastasis |
| IIIA1 | Positive retroperitoneal (pelvic and/or para-aortic) lymph nodes only |
| IIIA2 | Microscopic extrapelvic (above the pelvic rim) peritoneal involvement with or without positive retroperitoneal lymph nodes |
| IIIB | Macroscopic peritoneal metastasis beyond the pelvis up to 2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes |
| IIIC | Macroscopic peritoneal metastasis beyond the pelvis more than 2 cm in greatest dimension, with or without metastasis to the retroperitoneal lymph nodes (includes extension of tumor to capsule of liver and spleen without parenchymal involvement of either organ) |
| Stage IV | Carcinoma has spread beyond abdomen and to distant organs |
| IVA | Cancer cells in the pleural fluid |
| IVB | Spread to distant organs |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree