PET Imaging in Dementias and Extrapyramidal Disorders
Alfred Buck
Ehab M. Kamel
Klaus Tatsch
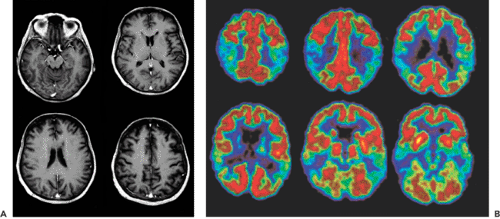
Some types of dementia display a distinct metabolic signature. The hallmark of Alzheimer disease is bilateral parietal hypometabolism, although the pathology can be more extended at later stages. Not affected are the motor and visual cortex, the basal ganglia, and the cerebellum. Pick disease is located more frontally, and multi-infarct dementia shows a patchy pathology. The main clinical indication for positron emission tomography (PET) scanning in dementia is the confirmation of Alzheimer disease (AD). This has become increasingly important with the introduction of cholinergic therapies. Before using an expensive medication, one would like to have a clear diagnosis. The tracer most often used is fluorodeoxyglucose (FDG). A typical example of an FDG-PET scan of an Alzheimer patient is shown in Figure 26.1.
Extrapyramidal disorders include idiopathic Parkinson disease (PD), progressive supranuclear palsy (PSP), multiple system atrophy (MSA), corticobasal degeneration (CBD), and DOPA-responsive dystonia. In most cases, the diagnosis is established clinically; however, sometimes the situation is not clear, and PET imaging can be helpful. All the extrapyramidal disorders are characterized by a disturbance of the dopaminergic system, which can be evaluated with various PET and single-photon emission computed tomography (SPECT) tracers. A summary of findings is presented in Table 26.1. In PSP, MSA, and CBD, the pre- and postsynaptic sides degenerate; in PD, the presynaptic side mainly is affected. This pattern allows separation of PD from the other extrapyramidal disorders.
Positron Emission Tomography Imaging in Dementias
Dementia is defined as a chronic, acquired, global, and progressive impairment of the intellect, memory, and personality without impairment of consciousness. The dementing diseases can be classified in two main categories: (a) diseases in which dementia is the only symptom (Alzheimer disease, Pick disease, multi-infarct dementia) and (b) diseases in which dementia is associated with other clinical or laboratory signs (infection, subdural hematoma, brain tumor, metabolic disorder, electrolyte imbalance, nutritional deficiency, Parkinson disease, Huntington disease, normal-pressure hydrocephalus). Because of its effect on the patient’s ability to function in society, dementia affects not only the patient but also the people in his or her environment. The prevalence of dementias increases with age (3% of the population older than 65 years, 10% of the people older than 75 years) (1), and dementing diseases will thus become a growing sociologic health problem in the aging society of developed countries. The number of affected elderly people is thought to have doubled between 1981 and 2001 (2).
Table 26.1 Pet and Spect Tracers for the Evaluation of the Dopaminergic System | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Indication for Positron Emission Tomography Studies
The diagnostic assessment in dementia includes a detailed history, a physical (especially neurologic) examination, blood and cerebrospinal fluid (CSF) screening, and computed tomography (CT) or magnetic resonance imaging (MRI). These tools are very useful in detecting most of the treatable causes of disorientation and loss of memory. For the remaining dementing diseases, such as neurodegenerative disorders and some cerebrovascular diseases, the definitive diagnosis is often based on biopsy or postmortem histopathologic findings. Functional imaging, such as positron emission tomography (PET), in combination with morphologic imaging, such as CT or MRI, helps in identifying the cause of dementia and in determining the neurophysiologic mechanisms underlying the disorder.
Normal Aging
Histopathologic findings show that the brain undergoes a number of age-related changes, which include neuron loss, ventricular enlargement, and cortical sulcal atrophy. CT and MRI are valuable techniques for identifying brain volume loss and ventricular enlargement. In an MRI study of 76 normal subjects, it was shown that the volumes of the cerebral hemispheres, of the frontal and temporal lobes, and of the amygdala-hippocampal complex decrease with age, whereas the ventricular volumes increase (3). The FDG-PET findings show a reduced metabolic rate in the elderly brain compared with that of young controls (4). However, when corrected for brain atrophy with CT or MRI, this age-related effect disappears (5). Effects due to atrophy should therefore always be taken into account when interpreting FDG-PET scans of elderly patients.
Alzheimer Disease
Alzheimer disease (AD) is the most common cause of dementia. The clinical diagnostic criteria require progressive, chronic cognitive deficits in patients aged 40 to 90 years without an identifiable underlying cause (6). Although these criteria permit accurate identification of AD patients with severe disease, it is very difficult to diagnose patients with early disease and to differentiate them from patients with other forms of neurodegenerative disorders.
Although the diagnosis is done on microscopic criteria, the brain of AD patients often shows characteristic changes on gross examination, such as cortical atrophy in the frontal, temporal, and parietal cortices, as well as frequent enlargement of the lateral and third ventricles (7,8). Analyzing the atrophy pattern in CT or MRI helps in distinguishing AD from normal age-related atrophy. With appropriate scan angles, it is possible to evaluate the temporal lobe and the hippocampus. The sizes of the sylvian fissures, the temporal horns, the temporal sulci, and the suprasellar cisterns characterize the temporal lobe atrophy and aid in differentiating brains affected with AD from normal brains (9). Furthermore, atrophy of the hippocampus present in AD patients is detected by an enlargement of the choroids–hippocampal fissure (10). Atrophy analysis by hand-drawn regions of interest, such as the hippocampus and the temporal role, helps in identifying AD, but this method is observer dependent (11,12).
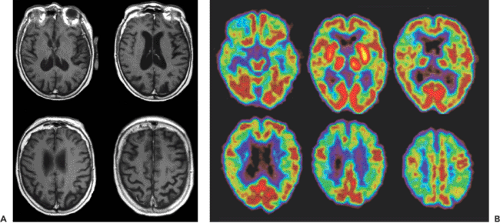
Different FDG-PET studies that compared AD patients with age-matched normal controls showed a 20% to 30% decrease in whole-brain FDG uptake, with prominent hypometabolism in the bilateral parietal and temporal lobes (13,14,15) (Fig. 26.1). The visual and motor cortices appear to be spared this reduced FDG uptake. Studies of the cerebral blood flow with H2O-PET or with technetium 99m [99mTc]hexamethylpropyleneamineoxime (HMPAO)-SPECT (single-photon emission computed tomography) show a similar picture, with relative hypoperfusion in the parietal and temporal lobes (16,17). The biparietal pattern is highly predictive for AD, but it is not always present. In a study involving 129 patients with dementia, Salmon et al. (18) found that 97% of clinically or pathologically diagnosed AD subjects had an abnormal FDG distribution, with 66% showing the typical bilateral pattern. After repeated scans, the patients with atypical patterns tended to evolve to the characteristic FDG distribution. With disease progression, the hypometabolism and the hypoperfusion extended to the frontal regions (15) (Fig. 26.2). The magnitude and extent of the hypometabolism has been shown to correlate with the severity of the dementia symptoms (19).
Different investigators showed that PET appears to be a valuable tool in predicting AD in patients with mild dementia (20). This clearly shows the utility and advantage of PET in the assessment of demented patients. Since the introduction of new therapies for AD, such as with cholinesterase inhibitors, early diagnosis may be of importance for the success of the treatment. PET also appears to be a good modality for assessing the effect of these new treatments. When treated with cholinesterase inhibitors, AD patients showed an increase in the impaired cerebral glucose metabolism (21).
Neuroreceptor imaging is a promising tool in the diagnosis of AD. Different studies identified alterations of the neurotransmission of the cholinergic, the serotonergic, and the γ-aminobutyric acid (GABA)-ergic systems (22,23,24). However, these types of PET examinations are not yet part of the clinical routine.
Multi-Infarct Dementia
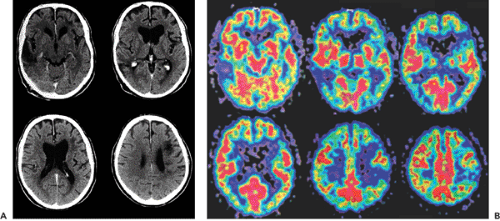
After AD, multi-infarct dementia (MID) is the second most common cause of dementia, accounting for about 13% to 24% of cases. MID is caused by multiple infarcts that are usually distributed asymmetrically throughout the cortex. Clinically, the symptoms tend to progress stepwise, in contrast to the constant disease progression in AD. CT and
MRI have been shown to be sensitive in detecting MID. Vascular dementia is supported by the demonstration of vascular lesions by T1-weighted MRI or CT (25,26,27). The absence of vascular lesions in these imaging modalities is sufficient to exclude a vascular etiology for the dementia. FDG-PET studies of patients with MID show multiple scattered focal areas of hypometabolism corresponding to the lesions seen on MRI (26,28) (Fig. 26.3). The severity of the dementia was found to correlate with the total volume of hypometabolic brain tissue. The lesions seen in PET appear to be greater than the corresponding MRI lesions and thus can be of help in early diagnosis. MID and AD commonly coexist, which makes it necessary to combine FDG-PET and MRI findings to establish the diagnosis (26).
MRI have been shown to be sensitive in detecting MID. Vascular dementia is supported by the demonstration of vascular lesions by T1-weighted MRI or CT (25,26,27). The absence of vascular lesions in these imaging modalities is sufficient to exclude a vascular etiology for the dementia. FDG-PET studies of patients with MID show multiple scattered focal areas of hypometabolism corresponding to the lesions seen on MRI (26,28) (Fig. 26.3). The severity of the dementia was found to correlate with the total volume of hypometabolic brain tissue. The lesions seen in PET appear to be greater than the corresponding MRI lesions and thus can be of help in early diagnosis. MID and AD commonly coexist, which makes it necessary to combine FDG-PET and MRI findings to establish the diagnosis (26).
Frontotemporal Dementia
Frontotemporal dementia (FTD) defines a group of dementing diseases characterized by atrophy of the frontal and the temporal lobes. Pick disease, the best known
member of this group, is a neurodegenerative disease associated with atrophy, neuronal loss, gliosis, and the presence of Pick bodies on histopathologic examination. These alterations affect predominantly the frontal and temporal lobe of patients in middle to late adulthood.
member of this group, is a neurodegenerative disease associated with atrophy, neuronal loss, gliosis, and the presence of Pick bodies on histopathologic examination. These alterations affect predominantly the frontal and temporal lobe of patients in middle to late adulthood.
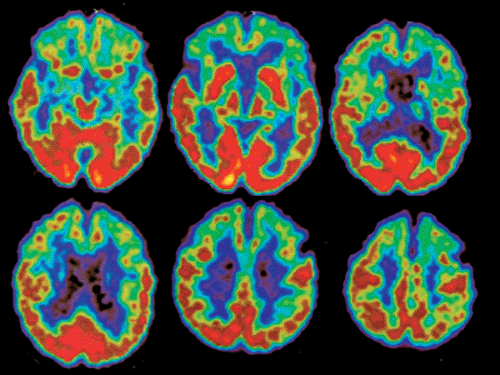 Figure 26.4 Pick disease: a 67-year-old paraspastic woman with dementia. FDG-PET shows bifrontal as well as anterior bitemporal lobe hypometabolism with normal temporoparietal FDG uptake. |
Clinically, the distinction between Pick disease and AD is difficult. Frontal or temporal atrophy, as well as widening of the interhemispheric fissure frontally, with compensatory enlargement of the frontal horn, can be seen in MRI or CT (29). Differentiation between AD and FTD can be difficult on the basis of these findings. In PET studies, subjects with Pick disease show a symmetric reduction of glucose metabolism in both frontal and anterior temporal lobes (30) (Fig. 26.4). These same regions appear also to have a reduced perfusion (31). This pattern aids in distinguishing this disease from AD, which is important because AD patients could benefit from specific treatment, in contrast to subjects with Pick disease. Other disorders, such as schizophrenia, also have bilateral frontal hypometabolism but are not difficult to differentiate clinically from Pick disease.
Normal-Pressure Hydrocephalus
Normal-pressure hydrocephalus (NPH) is an important cause of dementia because it is potentially treatable. Clinically, the triad of dementia, ataxia, and urinary incontinence characterizes this disease. Although there is a dilation of the ventricles, the cerebrospinal pressure appears to be normal. Although the clinical and radiologic diagnostic criteria are clear, in some cases, identification of NPH can be difficult. Making the correct diagnosis is important because the patient may benefit from a CSF shunt. The success of shunting is highest for patients with a history of meningitis or subarachnoid hemorrhage. MRI and CT findings in patients with NPH show variable grades of ventricular enlargement with rounding of the anterior third ventricle and increases in the size of the temporal horns and the sylvian fissures (32). A good parameter for hydrocephalus is the increased ratio of the maximum frontal ventricular horn width to the transverse inner diameter of the calvaria. A CSF flow void within the aqueduct and the third ventricle can be seen in MRI (33). PET studies done on NPH patients yield a diffuse cortical reduction of perfusion and metabolism without any regional predilection (34,35) (Fig. 26.5). The ventricular dilation, when massive, also can be identified with PET. The selection of patients for shunting is based on clinical, CSF, and imaging findings combined with response to spinal tap and removal of CSF.
Alcohol-Related Dementia
In patients with a long-term history of alcoholism, dementia can develop, along with impairment of orientation. In PET studies done on alcoholic patients, investigators found a decrease in metabolism in the whole brain but especially in the cortical and subcortical brain regions (36). Other studies reported more pronounced hypometabolism in the left hemisphere. Chronic alcoholic patients with cerebellar degeneration show hypometabolism in the superior vermis when compared with age-related control subjects. A study that compared the effects of administering alcohol to chronic alcoholics and normal subjects found that the former exhibited reduced glucose metabolism in the occipital, prefrontal, and cerebellar cortices, which are the regions with highest density of the GABAA receptors (37).
Creutzfeldt-Jakob Disease
Creutzfeldt-Jakob disease (CJD) is caused by a transmissible prion. The disease is characterized by a subacute spongiform encephalopathy in which a rapidly increasing dementia develops; the patients die shortly after diagnosis. Because this disease was not consistently recognized until recently, only a few PET studies with proven autoptic or bioptic diagnosis have been carried out. CJD patients show decreased FDG uptake, but the pattern is not yet clear. Perfusion also appears to be reduced (38).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree