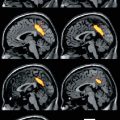
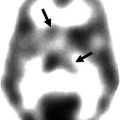
Fig. 35.1
Schematic representation of the rationale underlying the use of TSPO PET imaging to visualize the neuroinflammatory pathology in MS. The activation of microglia due to neuroinflammatory processes is associated to the upregulation of TSPO, leading to the increase of available TSPO for PET radioligands binding. The brain images on the right have been obtained with PET and [11C]PK11195 in a healthy volunteer (top) and a patient with MS (bottom) (Politis et al. 2012)
Table 35.1
Autoradiography studies using TSPO radioligands and correlation with immunohistochemistry markers of activated microglia or markers of demyelination in postmortem MS brain
Postmortem studies | Radioligand | Findings |
|---|---|---|
Benavides et al. (1987) | [3H]-PK11195 | Areas of high specific binding colocalized with demyelinating plaques within the white matter |
Vowinckel et al. (1997) | [3H]-PK11195 | Binding correlated with CD68-labeled activated microglia |
Banati et al. (2000) | [3H]-PK11195 | Areas of high specific binding colocalized with demyelinating plaques within the white matter and with oil red O-positive macrophages and EBM11-positive microglia |
Venneti et al. (2008) | [3H]-PK11195 | Binding correlated with CD68-labeled activated microglia |
[3H]-DAA1106 | ||
Owen et al. (2010) | [3H]-PK11195 | Areas of high specific binding colocalized with demyelinating plaques within the white matter |
[3H]-PBR28 |
PET Studies in MS Patients
The TSPO ligands, used to date in PET studies involving patients with MS, are [11C]PK11195, [11C]vinpocetine, [11C]PBR28, and [18F]FEDAA1106. [18F]PBR111 is being currently tested. The only well-characterized radiotracer is [11C]PK11195, which has been used for more than a decade. The other radiotracers have been only used in pilot studies so far, and only preliminary data on their application as tools for imaging in MS are available.
[11C]PK11195 Studies
Studies have showed an increased uptake of [11C]PK11195 in various neurological disorders other than MS, namely, HIV encephalitis, Alzheimer’s disease, Huntington’s disease, Parkinson’s disease, multiple system atrophy, stroke, amyotrophic lateral sclerosis, CNS neoplasms, and traumatic brain injuries (see reviews by Cagnin et al. (2007), Venneti et al. (2006)).
Seven [11C]PK11195 PET studies have been reported. The studies are summarized in Table 35.2. In general, areas with increased [11C]PK11195 uptake were found in MS brain compared to matched healthy controls. While in healthy volunteers the brain uptake of [11C]PK11195 was extremely low, in MS patients areas with increased uptake were consistently observed to correspond to white matter gadolinium-enhancing lesions (Banati et al. 2000; Debruyne et al. 2002, 2003; Vowinckel et al. 1997). Furthermore, some studies indicated that there were focal areas of increased uptake also in normal-appearing gray and white matter areas, indicating that TSPO increases, indicative of inflammatory processes, were present in white and gray matter areas which appeared normal on MRI (Banati et al. 2000). The overlap between MRI lesions and increased radioligand uptake was higher during relapses (Banati et al. 2000), consistent with the role of activated microglia in active acute MS lesions (Peterson et al. 2001). A positive correlation has been suggested between ligand uptake and disease duration, disability, and brain atrophy (Banati et al. 2000; Debruyne et al. 2003; Versijpt et al. 2005); however, the correlations were not consistently replicated across different studies. Also, the observed differences between MS patients and controls in [11C]PK11195 normal-appearing gray and white matter parenchymal binding have not been consistent across studies. Although the study by Banati found an increased radioligand uptake in the thalamus and brain stem of MS patients compared to healthy controls (Banati et al. 2000), in Debruyne’s study the authors did not find any between-group differences in the areas outside the lesions (Debruyne et al. 2003). Also, the latter study found a decreased uptake in T2 lesions compared to normal-appearing tissue, while an increased [11C]PK11195 uptake was associated with relapses. The authors suggested that upregulation of TSPO may be transient; however, issues related to different quantification methods may account for the differences found in this study compared to others.
Table 35.2
PET studies using TSPO radioligands in MS patients
Author | Subjects | PET ligand | Results |
|---|---|---|---|
Vowinckel et al. (1997) | 2 MS | [11C]PK11195 | High focal [11C]PK11195 binding in MRI-Gd-active lesion |
Banati et al. (2000) | 12 MS; 8 HV | [11C]PK11195 | Higher global and Gd-active lesions binding in MS relative to HV |
Debruyne et al. (2003) | 22 MS; 7 HV | [11C]PK11195 | No group difference in global [11C]PK11195 binding; higher binding in Gd-active lesions relative to normal-appearing brain |
Higher binding in T2 lesions of MS patients during relapses compared to HV. Positive correlation between [11C]PK11195 binding and disease duration | |||
Versijpt et al. (2005) | 24 MS; 8 HV | [11C]PK11195 | Positive correlation between [11C]PK11195 binding in normal-appearing white matter and brain atrophy |
Negative correlation between binding in T2 lesions and atrophy | |||
Vas et al. (2008) | 4 MS | [11C]PK11195 | Higher global uptake for [11C]vinpocetine relative to that of [11C]PK11195 |
[11C]vinpocetine | Differences between ligands in binding in peri-plaque region | ||
Oh et al. (2010) | 11 MS; 7 HV | [11C]PBR28 | No group difference in global [11C]PBR28 binding; higher binding in Gd-active lesions relative to normal-appearing brain |
Increased [11C]PBR28 binding preceded appearance of GD enhancement | |||
Ratchford et al. (2011) | 9 MS; pre- and post-glatiramer acetate treatment | [11C]PK11195 | Decrease in global and regional [11C]PK11195 binding after 1 year of glatiramer acetate treatment |
Politis et al. (2012) | 16 MS; 8 HV | [11C]PK11195 | Higher cortical gray matter [11C]PK11195 binding in SPMS relative to RRMS and HV |
In SPMS, positive correlation between cortical GM binding and MS disability |
Interestingly a recent study, which was specifically focused on cortical pathology, found an increase in cortical gray matter [11C]PK11195 uptake in MS patients relative to healthy controls, which was more pronounced in secondary progressive than relapsing–remitting patients (Fig. 35.2). There was a significant correlation between high cortical [11C]PK11195 uptake and disability as measured with EDSS, and this relationship was stronger for the subgroup of SPMS patients (Politis et al. 2012). The distribution of increased signal was not uniform across the cortical gray matter, and the cortical regions with highest increase included precentral and postcentral gyri. The uptake in the white matter was also increased in MS patients relative to controls; however, there was no relationship between disability and white matter uptake. This in vivo evidence of cortical involvement in MS pathology, predictive of MS disability, is consistent with recent neuropathological studies that described the presence of subpial gray matter demyelination secondary to the presence of substantial meningeal inflammation in some cortical sulci. Importantly, meningeal inflammation was associated to increased density of activated microglia and substantial neuronal loss in gray matter lesions of the precentral gyrus of SPMS patients (Howell et al. 2011; Magliozzi et al. 2007, 2010), which is consistent with the findings of the PET imaging evidence (Politis et al. 2012).
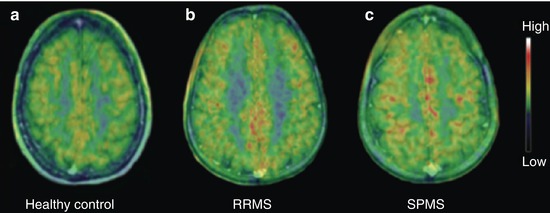

Fig. 35.2
[11C]PK11195 images indicating the TSPO density in the brain of (a) a healthy 40-year-old woman, (b) a 46-year-old man with a 12-year history of RRMS, and (c) a 46-year-old woman with secondary progressive multiple sclerosis (SPMS) and a 26-year history of MS
Only one longitudinal study has been conducted so far. The authors investigated the effects of 1-year treatment with glatiramer acetate, an immunosuppressive agent commonly used as a treatment of relapsing–remitting MS, on the uptake of [11C]PK11195 in nine previously untreated relapsing–remitting MS patients. A significant decrease was noted in cortical gray matter and cerebral white matter, and a trend towards decreased uptake was seen in the putamen and thalamus (Ratchford et al. 2011). However, these findings should be considered preliminary as the study did not include a control group and the sample size was small.
Despite these promising results, the use of [11C]PK11195 is limited by a low signal-to-noise ratio, secondary to low brain penetration and high nonspecific binding of the tracer; therefore, there has been great interest in the development on newer radioligands with improved characteristics.
Novel TSPO PET Radioligands
A study compared the uptake of [11C]PK11195 and that of the novel tracer [11C]vinpocetine in 4 MS patients. The global uptake of vinpocetine was approximately 50 % higher, reflecting greater brain penetration compared to [11C]PK11195. The regional and the peri-plaque uptakes were also investigated. The binding was higher for [11C]vinpocetine in the frontal lobe, thalamus, and peri-plaque regions. Interestingly, there was a weak correlation between the binding values of the two tracers within the same regions, and the two ligands’ behavior was also different in the peri-plaque region, perhaps indicating that the two ligands bind differentially to the TSPO (Vas et al. 2008). However, the limitations inherent to small sample size, the lack of a control group, and the choice of the reference region may have accounted for the discrepancies observed.
The recently developed TSPO radioligand [11C]PBR28 was applied to MS patients in a study conducted by Oh and colleagues, based on the promising characteristics of the tracer, i.e., high affinity for TSPO and high brain penetration (Oh et al. 2010). Eleven MS patients and seven healthy controls were studied, and a follow-up scan in six MS patients was also obtained. The results indicated that the parenchymal [11C]PBR28 binding did not differ between MS patients and healthy controls; however, an altered compartmental distribution of TSPO expression in the MS brain was suggested, based on a higher ratio of white matter to gray matter fraction of [11C]PBR28 binding in MS patients relative to controls. No relationship was observed between [11C]PBR28 binding and MRI-based lesion load. Focal increased binding was observed to correspond to Gd-enhancing lesions, and, importantly, the comparison between baseline and follow-up scans indicated that increased focal [11C]PBR28 binding preceded the development of some new gadolinium-enhancing lesions.
The results of a recent investigation using the novel tracer [18F]FEDAA1106 in 9 MS patients in acute relapse and 5 healthy controls have been recently reported (Takano et al. 2013). The results yielded by the study were negative, as significant differences were not found in any examined region of interest between patients and controls, and there was no evidence of any higher uptake in MRI-identified MS lesions relative to surrounding areas.
Other potential TSPO radiotracers that are promising for application in MS patients include [18F]PBR111, currently under investigation in MS patients at Imperial College; [11C]DAC and the SPECT tracer [123I]CLINDE, which have been successfully tested in the rodent model of MS, the experimental autoimmune encephalomyelitis; and [11C]DPA713 and [18F]PBR06, which are promising ligands tested in autoradiography assays on postmortem MS brain tissues (Owen et al. 2011a) and evaluated through a biomathematical model to predict their in vivo binding (Guo et al. 2012). There are a variety of other TSPO ligands which are currently tested in healthy subjects and for other neurological conditions; for a comprehensive review, see Chauveau et al. (2008).
Limitations of Current TSPO PET Tracers
Signal-to-Noise Ratio
The first and best-characterized TSPO tracer [11C]PK11195 is limited by a poor signal-to-noise ratio which complicates its quantification (Banati et al. 2000; Shah et al. 1994). This is a result of a combination of factors, the most significant of which is that [11C]PK11195 binds nonspecifically to sites in addition to TSPO. Moreover, [11C]PK11195 also suffers from low brain penetration (Debruyne et al. 2003; Vas et al. 2008). As a consequence of these factors, a very low signal from [11C]PK11195 is found in the normal brain, resulting in a poorly defined pattern of binding. It is also difficult to define normal reference tissue in diseases that are disseminated throughout the brain, such as MS.
Some of the second-generation TSPO radioligands that have been introduced offer higher specific to nonspecific signal. Using a biomathematical modeling approach based on in silico/in vitro data, it is predicted that the second-generation TSPO radioligands [18F]PBR111, [11C]PBR28, and [11C]DPA713 will perform better in vivo than [11C]PK11195 and that these radioligands will give greater power to detect group differences than [11C]PK11195, and as a result, fewer subjects may be required to detect mild-to-moderate TSPO increase in neurological populations (Guo et al. 2012).
Variation in Binding Affinities Using Second-Generation TSPO PET Radioligands
The use of second-generation TSPO radioligands, in particular [11C]PBR28, is complicated by the existence of apparent “non-binders,” subjects that appear not to bind [11C]PBR28. In vitro radioligand binding studies using postmortem human brain tissue revealed substantial population variation in the affinity of [11C]PBR28 for the TSPO. “Low affinity binders” (LABs) have a marked reduction in affinity for [11C]PBR28 (K i ~ 188 nM) in comparison to “high affinity binders” (HABs, K i ~ 3.4 nM). Hence LABs do not produce a measurable specific signal in PET studies with 11C-PBR28 and appear as “non-binders” (Owen et al. 2010). A third group was also identified (“mixed affinity binders” (MABs)), who express the HAB and LAB binding sites in approximately equal number (Owen et al. 2010). This variation in binding affinity is not specific to [11C]PBR28; all second-generation TSPO radioligands tested behave in a similar manner (Owen et al. 2011a). Whether also [11C]PK11195 binding varies between HABs, MABs, and LABs is not clear.
Interestingly, the variation in binding affinity was found to be due to a single nucleotide polymorphism (rs6971) in the gene encoding the TSPO (Owen et al. 2012), which causes a substitution of threonine and alanine at position 147 (Ala147Thr). This substitution is common in Caucasians: 49 % of subjects are Ala–Ala (HABs), 42 % are Ala–Thr (MABs), and 9 % are Thr–Thr (LABs), but its prevalence varies across ethnic groups. Since the binding class is consistent within each subject for all the second-generation ligands tested to date, genotyping the rs6971 polymorphism can help the quantitative assessment of TSPO ligand binding, by allowing binding affinity to be predicted at screening.
Despite that the possibility of predicting the classes of binding affinity at screening is very important for the application of second-generation ligands in basic and pharmacological research, the presence of a variation in binding affinity across the population may still be impractical for the clinical application of these tracers. For example, genotypization would be necessary in all the patients undergoing PET imaging with [11C]PBR28, in order to distinguish those who are HABs from those who are MABs, and in approximately 10 % of the population (LABs), the specific signal would not be measurable in any case.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree