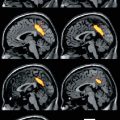
Fig. 42.1
(a) Gadolinium enhanced T1-weighted MRI revealing recurrence of an anaplastic oligoastrocytoma. (b) 99mTc-Tetrofosmin brain SPECT revealing increased tracer uptake in the corresponding area
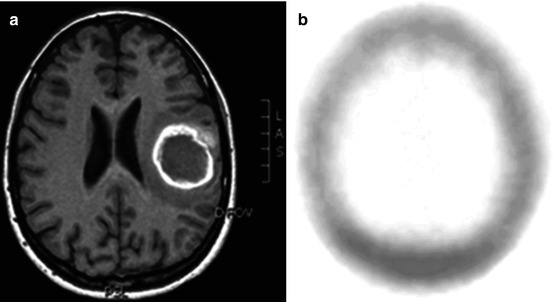
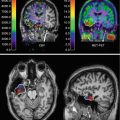
Fig. 42.2
(a) T1-weighted MRI in a patient with an intracerebral hemorrhage. The neoplastic hemorrhagic origin could not be ruled out. (b) Brain SPECT demonstrating no tracer uptake suggesting nonneoplastic hemorrhagic origin
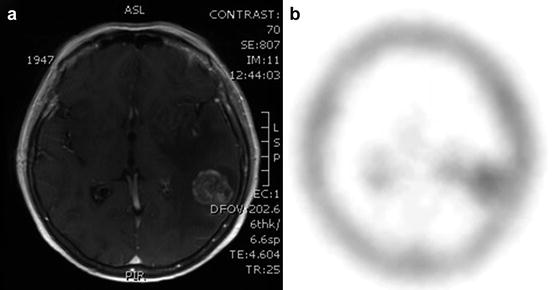
Fig. 42.3
(a) T1-weighted MRI after gadolinium infusion in a patient with a metastatic lesion. (b) 99mTc-Tetrofosmin brain SPECT revealing increased tracer uptake in the corresponding area
Primary brain tumors can be benign or malignant. According to the World Health Organization (WHO) grading system, they are classified into four grades (I–IV). There are many types of primary brain tumors; meningiomas are the most common, accounting for 34 % of all, followed by gliomas (32 %). Glioblastoma (WHO Grade IV) is the most malignant primary brain tumor, accounting for 16.7 % of all tumors. Other types are pituitary adenomas (13.1 %), neuroepithelial tumors (5.1 %), lymphoma (2.4 %), oligodendrogliomas (2 %), ependymomas (1.8 %), embryonal tumors (1 %), and craniopharyngioma (0.7 %).
Causes and risk factors can be environmental and genetics. The most common presenting symptoms of brain tumors are due to increased intracranial pressure (headache, nausea, vomiting), seizures, focal neurological deficits, and possibly cognitive deterioration (Perry and Schmidt 2006). Treatment depends on tumor aggressiveness. Radical surgical excision is usually curative in benign tumor. Treatment options for high-grade lesions are surgery, radiation therapy, and chemotherapy. The majority of patients get a combination of treatments (Alexiou et al 2009).
With regard to imaging, the most commonly used methods for diagnosis and follow-up are magnetic resonance imaging (MRI) and computed tomography (CT). Nevertheless, in patients with brain lesions, it is not uncommon for the above modalities to provide nonspecific information, even after contrast administration. Advanced MRI techniques, including diffusion, perfusion, and spectroscopy, offer important diagnostic advantages over conventional imaging in the assessment of patients with brain tumors. In fact, proton magnetic resonance spectroscopy (H-MRS) provides additional information on the metabolic composition within an area of a tissue, by comparing the relative concentration of several metabolites. It is currently being extensively evaluated towards the distinction of tumor recurrence from radiation necrosis and is considered the gold standard (Hollingworth et al. 2006; Amin et al. 2012). Imaging modalities used in nuclear medicine, namely, positron emission tomography (PET) and single-photon emission computed tomography (SPECT), have also been employed towards the evaluation of brain tumors. At present, PET constitutes the most sophisticated modality of nuclear medicine imaging for brain tumor evaluation, owing to higher resolution compared to SPECT. Nevertheless, SPECT has a lower cost, worldwide availability, and gained practical experience. The more sophisticated hybrid SPECT–CT scanners provide both functional and anatomic imaging to be acquired simultaneously (Hasegawa et al. 2002). Various SPECT radiotracers have been used to date and are presented in detail.
42.2 SPECT Radiotracers
Various SPECT radiotracers have been evaluated towards noninvasive assessment of brain lesions, with image acquisition usually performed 20 min after intravenous radiotracer administration. Among them, Thallium-201 (201Tl) was one of the first tracers that were widely employed (physical half-life 73 h, emitting γ-rays at 135 and 167 keV, and X-rays at ~70–80 keV, administered activity 148 MBq). The precise cellular uptake process is still questioned, but the sodium-potassium ATPase pump is probably involved, at least in part.
Technetium-99m-labeled compounds (physical half-life 6 h, emitting γ-rays at 140 keV) have also been studied and were proven advantageous over 201Tl due to higher photon flux, better spatial resolution, and less radiation burden to the patient (Benard et al. 2002; Soler et al. 1998). Among them, 99mTc-hexamethypropyleneamine-oxime (HMPAO) (administered activity 1,110 MBq) and more extensively 99mTc-hexakis-2-methoxy isobutyl isonitrile (99mTc-Sestamibi or 99mTc-MIBI) and 99mTc-Tetrofosmin (99mTc-TF) (administered activity 700 MBq for both) have also been studied. 99mTc-MIBI diffuses passively into the cell through the intact cellular membrane, driven by the membrane negative electric potential, and an estimated 95 % of the tracer is localized in the mitochondria. 99mTc-TF enters viable cells similar to Sestamibi and mostly localizes within the cytosol, with only a small fraction passing into the mitochondria. Although both 99mTc-TF and 99mTc-MIBI have been found in vitro to be substrates of P-gp, the fact that 99mTc-TF uptake in glioma cells is not particularly influenced by the expression of their MDR protein genotype substantiates a plausible clinical superiority over 99mTc-MIBI, which is prone to extracellular excretion by P-gp. Pentavalent Tc-99m dimercaptosuccinic acid (99mTc-(V)DMSA) (administered activity 700 MBq) is another tumor-seeking SPECT tracer, being a possible PO43- anion analogue. Its proliferative imaging potential in gliomas has been suggested in vitro and sporadically reported in vivo. Tsiouris et al. stated that mounting evidence indicates that 99mTc-(V)DMSA is a credible noninvasive proliferation depicter and its cellular accumulation linked closely to phosphate uptake and kinase pathway activation (Denoyer et al. 2004, 2005; Tsiouris et al. 2007; Amin et al. 2012). 99mTc-methionine and 99mTc-glucoheptonate have also been utilized, but are not easily available and not commonly used.
Radiolabeled amino acids have been evaluated and proved tracers for the delineation of tumors, particularly brain neoplasms. These tracers are not taken up by normal brain and provide a greater target-to-background contrast, thus allowing better characterization and differentiation of the tumor from the normal brain. L-3-[Iodine-123]-iodo-alpha-methyl-L-tyrosine (123I-IMT) is well established for SPECT imaging of gliomas, and p-[Iodine-123]-iodo-L-phenylalanine (123I-IPA) has been also used. The latter may prove promising for therapeutic use in gliomas after labeling with Iodine-131 (Hellwig et al. 2008).
42.3 Clinical Applications
The primary role of SPECT in brain tumor patients lies on the noninvasive assessment of tumor aggressiveness, differentiation of treatment-induced necrosis from tumor recurrence, assessment of response to treatment, and estimation of overall prognosis.
42.3.1 Characterization of Intracranial Masses: Differentiation of Brain Tumors from Nonneoplastic Lesions
The noninvasive assessment of the nature of an intracranial lesion is of paramount importance for patient’s management. Differentiation of high-grade from low-grade gliomas may not be always possible on conventional MRI, since non-enhancing high-grade gliomas exist. Furthermore, a ring-enhanced lesion may be a brain tumor, an abscess, tumefactive multiple sclerosis, or other nonneoplastic pathologies such as an intracerebral hemorrhage. The above problems are encountered in everyday practice.
Recently, Fotopoulos et al. studied by 99mTc-TF brain SPECT 106 cases with intracranial lesion suspicious of tumor on conventional radiological imaging. Ninety patients suffered from neoplastic lesions and 16 harbored nonneoplastic pathologies (abscesses, nonmalignant hemorrhages, radiation necroses). A lesion-to-normal (L/N) tracer uptake ratio of 2.8 could differentiate high-grade gliomas from low-grade lesions (Fotopoulos et al. 2011). Similar findings have been reported by other authors (Choi et al. 2000). Furthermore, 99mTc-TF differentiated effectively high- and low-grade gliomas from nonneoplastic lesions. Nonetheless, differentiation between primary gliomas (low or high grade) and metastases was not feasible on the basis of quantitative scintigraphic criteria.
Thallium-201, one of the most commonly used tracers for brain tumor imaging, showed promise for the differentiation of malignant from benign brain tumors. 201Tl brain SPECT could increase confidence in the diagnosis of intracranial lesions with ringlike contrast enhancement when MRI was not able to differentiate between benign and malignant disease. A brain tumor would show avid tracer uptake compared to the low uptake of a brain abscess (Kita et al. 2007). Another important issue that has been addressed is the optimal lesion size for an accurate diagnosis. In a study comparing 201Tl uptake between brain lymphoma and abscess, the authors reported that lesion size is a significant determinant of the accuracy of this tracer, which should be the initial diagnostic tool for lesions over 2 cm (Young et al. 2005). Furthermore, the location of a lesion is important, since infratentorial lesions may tend to display lower tracer uptake (Scott et al. 1994).
99mTc − (V)DMSA has been proven valuable for brain tumor characterization. Hirano et al. compared 99mTc-(V)DMSA and 201Tl in 100 patients with various brain pathologies. They found that early uptake ratios of both radiopharmaceuticals were closely related to tumor vascularity, but could not differentiate benign from malignant disease. The delayed tracer uptake ratio, retention ratio, and retention index were higher in malignant tumors than benign ones on 99mTc(V)-DMSA; however, there was no statistically significant difference between benign and malignant tumors on 201Tl. They concluded that 99mTc(V)DMSA is superior in imaging primary and metastatic brain tumors and differentiating their histological malignancy grade noninvasively (Hirano et al. 1997a).
99mTc − MIBI has been evaluated in various brain pathologies. If performed within 5 days of symptom onset, it has been found capable to differentiate neoplastic from nonneoplastic intracerebral hematomas (Minutoli et al. 2005). When compared to 201Tl, it proved superior for differentiating intracranial lymphoma from nonmalignant lesions, exhibiting similar sensitivity but higher specificity (Naddaf et al. 1998).
Somatostatin receptors (SSRs) are expressed in 70–100 % of meningiomas and can be used to image the radiolabeled tumors for maximizing resection, detection of residual, or early recurrent tumors or for therapeutic purposes (Dammers et al. 2009). Brain SPECT by the radiolabeled SSR-agonist peptide indium-111 (111In)-pentetreotide (111In-DTPA-octreotide) may provide important information for the noninvasive differentiation of meningiomas from other cranial dural-based pathology in conjugation with conventional MRI. This method provided high-negative predictive value (100 %) for the diagnosis of meningiomas (Nathoo et al. 2007). Yttrium-90 (90Y)-DOTA-Phe1-Tyr3-Octreotide (90Y-SMT 487, OctreoTher™) has shown potential for effectively treating patients with neuroendocrine tumors (Bushnell et al. 2004).
42.3.2 Differentiate Glioma Recurrence from Treatment-Induced Necrosis (TIN)
Malignant gliomas are the most common type of primary brain tumors and carry a dismal prognosis. Glioblastoma multiforme (GBM) usually has a rapid and fatal clinical course. The standard of care for newly diagnosed GBM includes surgical resection when possible, followed by radiotherapy and concomitant and adjuvant temozolomide. Nevertheless, high-grade gliomas usually recur despite treatment, whereas radiotherapy may cause postradiation damage (Alexiou et al. 2009). The incidence of treatment-related necrosis in GBM patients treated with concomitant temozolomide and radiotherapy reaches 30 % (Brandes et al. 2008). Differentiation between TIN and recurrent glioma on the basis of CT and MRI is not always possible. Nuclear medicine molecular imaging has also been actively implicated in the detection of recurrent tumor by SPECT and PET. Among the SPECT tracers, the most extensively studied are 201Tl and 99mTc-MIBI.
Recurrent disease exhibits increased 201Tl uptake compared to necrosis. Early and delayed postinjection imaging has been used to calculate the radiotracer retention index (RI) (i.e., the ratio of delayed L/N uptake ratio to early L/N uptake ratio); RI has been suggested as a significant diagnostic marker by Matsunaga et al. (2013). Even in the follow-up of low-grade gliomas by 201Tl, a tracer uptake index value of 1.25 as cutoff for detecting recurrent tumor activity resulted in 90 % sensitivity and 80 % specificity (Gomez-Rio et al. 2004).
99mTc − MIBI has also been extensively evaluated, with good reported results and a significant diagnostic advantage over contrast-enhanced MRI (Soler et al. 1998). In a study that included 81 patients, the sensitivity for tumor recurrence was 90 %, specificity 91.5 %, and accuracy 90.5 % (Le Jeune et al. 2006a). H-MRS proved equal to 99mTc-MIBI SPECT with 90 % sensitivity, 100 % specificity, 93 % accuracy, 83 % negative predictive value, and 100 % positive predictive value (Palumbo et al. 2006).
Apart from 201Tl and 99mTc-MIBI, 99mTc-TF has been also evaluated towards the detection of recurrent tumor, having the advantage of not being influenced by glioma MDR-phenotype (P-gp expression). A cut-off L/N value of 4.8 was found as most accurately discriminating recurrence from postirradiation damage (Alexiou et al. 2007). In a comparative study between relative cerebral blood volume (rCBV) by perfusion MRI and 99mTc-TF brain SPECT, they were found of similar diagnostic accuracy. Furthermore, a significant correlation between tracer uptake ratio and rCBV was verified. However, it must be kept in mind that 99mTc-TF SPECT exhibits suboptimal sensitivity in the detection of recurrent tumors located supratentorially in the posterior fossa (Barai et al. 2003).
99mTc − (V)DMSA also proved useful for the detection of glioma recurrence. In a comparative study between 99mTc-(V)DMSA SPECT and H-MRS, the first was more accurate for the detection of tumor residual tissues or recurrence in glioma patients with previous radiotherapy. 99mTc-(V)DMSA allowed an early and noninvasive differentiation of residual tumor or recurrence from TIN (Amin et al. 2012).
Imaging protein synthesis with the use of radiolabeled amino acids is another approach that has been employed towards the noninvasive detection of tumor recurrence. Amino acid uptake in normal brain tissue is low, thus recurrent gliomas can be readily distinguished from their surrounding normal tissue. The majority of labeled amino acid tracers have been developed for PET imaging. 123I-IMT has been validated in SPECT studies. Samnick et al. studied 78 glioma patients after primary therapy and possible tumor recurrence and found that 123I-IMT had 100 % sensitivity for the detection of high-grade glioma recurrence and 84 % and 92 % for Grade II and Grade III glioma recurrence, respectively (Samnick et al. 2002). In a comparative study between single-voxel H-MRS at 3.0 Tesla and 123I-IMT SPECT, a tracer uptake cutoff value of 1.62 proved superior to MRS (sensitivity 95 %, specificity 100 %, and accuracy 96 % versus 89, 83, and 88 %, respectively (Plotkin et al. 2004).
42.3.3 Assessment of Glioma Aggressiveness
Assessing the proliferative potential of tumor cells is of paramount importance for predicting their biological behavior, response to therapy, and prognosis. Noninvasive imaging modalities that could reliably assess the proliferative potential of intracranial space-occupying lesions in vivo would be of obvious significance (Alexiou et al. 2010a). Various methods have been proposed to estimate proliferation in tissue samples. The most extensively studied are flow cytometry, bromodeoxyuridine (BrdU) labeling index, MIB-1 antibody staining to the nuclear antigen Ki-67, staining to the proliferating cell nuclear antigen (PCNA), and argyrophilic staining to nucleolar organizing regions (AgNOR) (Prayson 2005). Among these, the MIB-1/Ki-67 assay can be easily applied, is considered as the most reliable method, and is performed routinely.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree