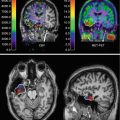
Fig. 23.1
Typical MR image of a BAVM
23.2.2 Natural History
After the clinical presentation of a BAVM, the most important issue is the estimate of the natural disease course during the rest of the life of the patient. The choice of treatment modality is dependent on this estimate, as the success rate and the risk of complications of the procedure should outweigh the risk of the BAVM in its natural history. However, studying the natural history poses a problem, since true observation prescribes the complete withdrawal of medical intervention, while medical ethics mandate every patient to be entitled to the best available care. Nevertheless, many studies have been performed over the decades, since in older days conservative management was the only treatment available.
The retrospective literature reports from the late twentieth century by Graf, Crawford, and Brown et al. (mean follow-up 4.8–10.2 years) revealed an overall hemorrhage risk of 2–3 % per year regardless of the mode of presentation (ref). In contrast to Crawford and Brown, the report by Graf pointed out a doubled hemorrhage risk during the first year following presentation with a bleeding. None of these studies revealed a predictor for a differentiated natural history (Brown et al. 1988; Crawford et al. 1986; Graf et al. 1983).
Ondra et al. reported the first prospectively collected data in 1990, describing a series of 166 unoperated symptomatic patients with a mean follow-up of 23.7 years. The constant rate of (recurrent) hemorrhage was 4.0 % per year, with a combined incidence of major morbidity and mortality of 2.7 % (annual mortality rate 1.0 %). There was no difference in the frequency of bleeding or death regardless of the way of presentation. Nevertheless, despite its design, this study also had significant limitations: the study inclusion dates were between 1942 and 1975, well before tomographic brain imaging was available, and in line with currently known annual hemorrhage rates it is estimated that there has been a considerable under-detection of cases; probably only one-third of the cases were identified (262 reported versus 825 estimated) (Ondra et al. 1990).
Nowadays, the knowledge of the natural history of BAVMs is dictated by large prospectively kept databases, e.g., the Columbia AVM Databank, the Kaiser Permanente Medical Care Program Cohort, the Scottish Intracranial Vascular Malformation Study, and the Toronto BAVM Study Group. At the time of the 2006 publication by Stapf et al., the Columbia database covered 622 consecutive patients that during follow-up had an average annual hemorrhage rate of 2.8 %. However, increasing age, hemorrhagic presentation, deep brain location, and exclusively deep venous drainage were recognized as independent predictors for hemorrhagic events during natural history follow-up. Without risk factors the average annual hemorrhage risk was calculated 0.9 %, while all risk factors together show a cumulative hemorrhage risk of 34.3 % per year (Stapf et al. 2006). In 2009, Da Costa et al. reported the Toronto database to contain 759 patients, of which 678 patients had sufficient information for analysis. He calculated an overall annual hemorrhage rate of 4.6 % for the entire cohort during follow-up. However, he as well found hemorrhagic presentation to be a significant independent predictor of future hemorrhage, whereas the presence of associated aneurysms and deep venous drainage showed a trend toward significance (da Costa et al. 2009). Based on these figures, the Columbia group launched a global multicenter randomized controlled trial aiming to include 800 adult patients with an unruptured BAVM to further elucidate its natural history (Fiehler and Stapf 2008).
23.2.3 Treatment Options
If conservative management of a BAVM is considered having a higher overall risk compared to active treatment, then several modalities are available. The main goal of BAVM treatment is the prevention of hemorrhage, which can be achieved by microsurgical resection of the nidus, endovascular embolization, radiosurgery, or a combination of these techniques.
Typical for a BAVM is that the brain tissue within the nidus is either absent or highly gliotic and nonfunctional (Anderson and Korbin 1958). This feature is eminent for a complete removal of the nidus without the risk for neurological complications. Partial treatment (obliteration less than 90 %) has been reported to be insufficient to prevent hemorrhage, although partial endovascular embolization targeted toward intranidal aneurysms and larger fistulas may be beneficial (Meisel et al. 2002; Wikholm et al. 2001).
The selection of the optimal treatment modality is usually made by multidisciplinary consensus and based on the experience of the local treating team, for which recommendations have been published by the American Stroke Association (Ogilvy et al. 2001). These recommendations are based upon classification by the Spetzler-Martin grading scale that appreciates the size of the nidus, the pattern of venous drainage, and the eloquence of the surrounding parenchyma (Table 23.1).
Table 23.1
Spetzler-Martin grading scale for bavms. The grade is calculated by adding up the points for each feature (Spetzler and Martin 1986)
Feature | Appreciation |
|---|---|
Nidus size | <3 cm – 1 point |
3–6 cm – 2 points | |
>6 cm – 3 points | |
Venous drainage | Superficial – 0 point |
Deep – 1 point | |
Nidus location | Not eloquent – 0 point |
Eloquent – 1 point |
ASA Recommendations
Surgical extirpation should be considered the primary mode of therapy for low-grade BAVMs (Spetzler-Martin grades I and II). Radiosurgery should be considered for small lesions when surgery may be associated with increased risk based on anatomical location or angioarchitecture.
Multimodality treatment with endovascular embolization followed by surgical resection may be used in intermediate-grade BAVMs (Spetzler-Martin grade III).
Invasive treatment of high-grade BAVMs (Spetzler-Martin grades IV and V) is associated with a high risk. Such BAVMs are generally managed conservatively.
Of course treatment decisions should be made on an individual basis and based on locally available expertise. For example, in the Toronto Western Hospital, single-compartment BAVMs (with a low Spetzler-Martin grade) are primarily treated by endovascular means.
The management of BAVM-associated aneurysms is a matter of debate and dependent on their size and location. We do not advocate the treatment of pre-nidal aneurysms as they are flow-related and regress following obliteration of the brain AVM. Intranidal aneurysms, on the other hand, are often the source of hemorrhage in acutely ruptured brain AVMs and may therefore represent an angioarchitectural point of weakness in an unruptured AVM warranting treatment.
23.3 Cerebral Proliferative Angiopathy
In 1989, Lasjaunias et al. launched the term cerebral proliferative angiopathy (CPA) for a peculiar type of large cerebral vascular malformations that demonstrated distinctive angiographic and clinical features discriminating it from a classical BAVM. In the Bicêtre neurovascular database of 1,434 BAVMs, a subgroup of 49 patients (3.4 %) with CPA was identified. It is more common in females (2:1) and presents at a mean age of 20 years (Lasjaunias et al. 2008).
Similar to a BAVM, CPA presents as a diffuse network of densely enhancing vascular although distinct angiographic characteristics:
Discrepancy between the large size of the nidus and a relatively small shunting volume
Absence of flow-related aneurysms
Presence of diffuse angiogenesis
Small caliber of a multitude of arteries and draining veins
The most striking difference between CPA and a BAVM is the existence of normal neural tissue intermingled with the abnormal vascular channels in CPA (Fig. 23.2). Since normally functioning brain is interspersed with abnormal vascular channels, the risk of neurological deficit in the surgical, radiosurgical, or endovascular obliterative treatment of CPA is highly increased compared to BAVM (Chin et al. 1992).
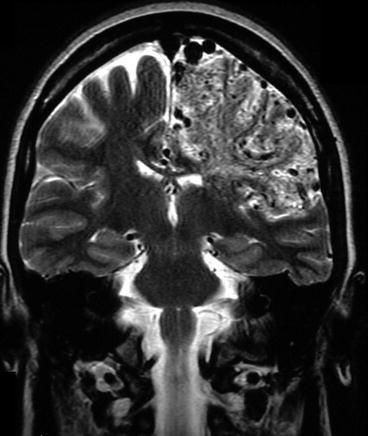

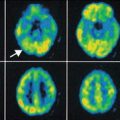
Fig. 23.2
The diffuse aspect of CPA on MRI with its characteristic lobar involvement
23.3.1 Clinical Presentation
Seizures, headaches, and transient ischemic attacks are far more frequent in CPA than in classical AVMs, while hemorrhages are exceedingly rare. Patients may present with progressive neurological deficits or with TIA-like symptoms indicating that hypoperfusion may be an important underlying pathological mechanism (Lasjaunias et al. 2008).
23.3.2 Natural History
The pathophysiology of CPA is unknown, but it is assumed to be an acquired condition induced by tissue hypoxemia related to local hypoperfusion. As such, transdural supply is a well-known feature of CPA, likely to be provoked by local hypoxemia. Ducreux et al. applied perfusion-weighted MRI in seven CPA patients to demonstrate hypoperfusion throughout the affected hemisphere (Ducreux et al. 2004). Therefore, stroke-like symptoms, e.g., TIAs and nonhemorrhagic neurological deficits, are likely to occur during the natural disease course. Hemorrhage occurs much less frequently as a presenting symptom than in a BAVM, but once a hemorrhage has occurred, there is a high risk for further hemorrhages (Lasjaunias et al. 2008).
23.3.3 Treatment Options
Since the typical gliotic plane of a BAVM nidus with the surrounding normal white matter is nonexistent in CPA, the surgical resection or endovascular embolization of CPA is impossible without damaging healthy brain parenchyma. As such, obliterative treatment possibilities of CPA carry a high risk of neurological deficits. In patients presenting with hemorrhages, partial targeted embolization may be employed if a target (i.e., an intranidal aneurysm) can be identified. Since the pathological mechanism is related to local hypoxemia, treatment should be targeted at increasing the blood supply to the affected hemisphere. Both in the series of Lasjaunias and in a recent case report by Ellis et al., the treatment of these lesions by pial synangiosis (burr hole treatment) leads to an increase in blood supply to the affected hemisphere and an amelioration of symptoms (including seizures, headaches, and TIA-like symptoms) (Ducreux et al. 2002a; Ellis et al. 2011).
23.4 Radiological Imaging
The differentiation between BAVM and CPA has large implications for the choice of the management strategy. Since it is not always possible to diagnose CPA on clinical grounds in the individual patient but pathological specimens have proven a clear anatomical and functional difference between the disorders, determination is largely dependent on imaging techniques.
23.4.1 Cross-Sectional Imaging
With CT and MRI the presence of a vascular malformation in the brain is well identifiable, but further discrimination between BAVM and CPA may be problematic. On CT and MRI, CPA presents as a diffuse network of densely enhancing vascular spaces intermingled with normal brain parenchyma. T1- and T2-WI show small, widely distributed flow voids that may involve multiple lobes or the entire hemisphere. In most cases, the primary lesion extends from the surface into the basal ganglia and thalamus and involves more than one vascular territory. Compared to the size of the nidus, relatively few draining vessels are seen on CT or MRI, and these are only moderately enlarged. Perfusion-weighted MRI (p-MRI) demonstrates perfusion abnormalities far beyond the boundaries of the morphological malformation seen on conventional MR sequences, so the disease actually affects the entire hemisphere. The nidus shows increased cerebral blood volume, slightly decreased TTP (time to peak), and prolonged mean transit time (MTT). Remote from the nidus, in normal-appearing cortical and subcortical areas, the TTP is increased and the blood volume is decreased, indicating remote, widespread hypoperfusion. Lasjaunias et al. reported that in the majority (67 %) of CPA cases, its localization was in between the different vascular territories (watershed zone). In addition he noticed that in comparison with the size of the CPA nidus, there was a relative paucity of draining veins that were often only moderately enlarged (Lasjaunias et al. 2008). Another finding indicative of CPA is that both on CT and MRI, most vessels densely enhance with intravenous contrast because of relatively slow flow, while in BAVM, most vessels do not enhance due to the high flow and turbulence (Vargas and Castillo 2011).
A BAVM usually has a more or less compact nidus (Fig. 23.3). T2-WI shows punctate to linear flow voids in the subarachnoid space over the brain reflecting the dilated surface vessels. Within the brain, the punctate flow voids often lie within hyperintense, gliotic parenchyma. This gliosis may extend to the surrounding brain tissue. T1-WI shows highly variable signal within the AVM, due to turbulent flow, blood degradation products, and the variable flow rate in the veins. Different from CPA, the perfusion abnormalities do not extend far beyond the boundaries of the nidus (Ducreux et al. 2002b, 2004; Vargas and Castillo 2011) (Lasjaunias et al. 2008). Recent studies by the Toronto group with BOLD-MRI have demonstrated a severely impaired cerebrovascular reserve in patients with CPA, which was also demonstrated in BAVMs in combination with seizures, although functional imaging with the BOLD technique in the past by Ducreux et al. failed to point out the functional aspect of the brain tissue in between the CPA nidus (Ducreux et al. 2002a; Fierstra et al. 2010, 2011).
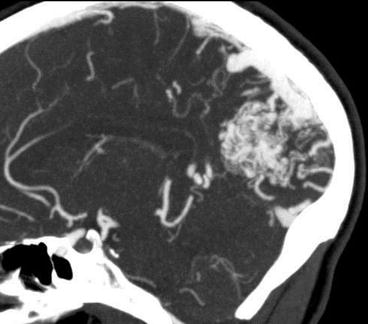

Fig. 23.3
CTA can depict the compact nidus of a BAVM but lacks the dynamic features
23.4.2 Cerebral Catheter Angiography
Lasjaunias et al. described the characteristics of cerebral catheter angiography in CPA in his launching paper in 2008. In BAVM pathognomonic dominant arterial feeders are present, while CPA is fed by multiple arteries that are not enlarged or are only moderately enlarged. Unlike classical AVMs, all arteries of the affected region contribute equally to the malformation. Stenosis of the proximal arteries is present in 40 % and affects the internal carotid artery (ICA) and the proximal horizontal segments of the middle cerebral artery (M1) and anterior cerebral artery (A1). Flow-related proximal aneurysms can be present in BAVM but are not seen in CPA. The nidus has a classical appearance with scattered areas of “puddling” of contrast within what looks like capillary ectasias (Fig. 23.4). These persist into the late arterial and early venous phase. The nidus is usually fuzzy, poorly circumscribed, and >6 cm in diameter. Intranidal vessels show a capillary ectasia. Perinidal angiogenesis is often present and difficult to distinguish from the nidus proper. There is no high-flow fistulous component to the arteriovenous shunt, so early opacification of draining veins is uncommon. The size of the draining veins, the “shunt volume,” and the time until the veins are visualized never “correspond” to the size of the nidus. CPA has a higher tendency for transdural supply from the external carotid circulation than BAVM (Lasjaunias et al. 2008).
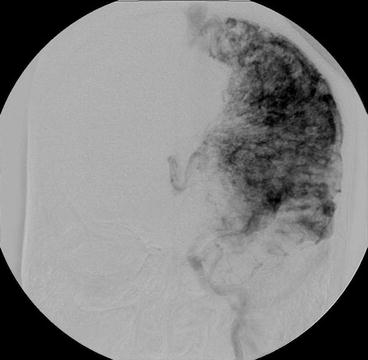

Fig. 23.4
Classical appearance of a CPA nidus on conventional X-ray angiography
23.5 PET Imaging of Brain AVM
The current classifications of BAVM are based on surgical anatomical features, such as the location and size of the nidus and the pattern of the venous drainage. However, as these attributes are mainly focused on the difficulty for interventional assessment of the vascular lesion, they do not allow for a functional-dynamic differentiation between a genuine BAVM and CPA. Nevertheless, as stated before, the distinction between BAVM and CPA is of great importance, since there are different therapeutic indications. With conventional radiological imaging techniques, i.e., CT, MRI, and angiography, it is difficult to distinguish between BAVM and CPA. PET imaging though can be a useful adjunct, allowing for visualization of specific molecular processes, i.e., processes that are different in BAVM and CPA.
To date, PET imaging of BAVM has mainly been focused on the hemodynamic and metabolic effects of BAVMs. The first study on PET imaging of BAVM was published in 1977 and was a case report on a patient with a BAVM in the right posterior frontal region of the brain. Using [68Ga]-EDTA an early focal filling of the radioligand was found in the right frontal region, which was followed by a deep spreading pattern. The average distribution of the radioligand showed a rapid blood flow rate in the nidus as well as in adjacent tissue. The blood flow rate was (visually) found to be impaired in the area behind the nidus (Yamamoto et al. 1977).
After this first study on BAVM in the early days of PET research, most of the later PET studies focused on imaging of the cerebral blood flow (CBF), the cerebral blood volume (CBV), the cerebral metabolic rate for oxygen (CMRO2), the oxygen extraction fraction (OEF), and cerebral metabolic rate for glucose (CMRGlc) using the radioligands [15O]H2O, [15O]O2, [15O]CO2, [15O]CO, and [18F]FDG (summarized in Table 23.2).
Table 23.2
Pet studies on metabolic and hemodynamic parameters in bavm
Measure | Author | PET tracer | Finding | Remark | |
|---|---|---|---|---|---|
CBF | Sollevi et al. (1987) | [15O]H2O | ↓/= | Perifocal to BAVM | Patients were under anesthesia during PET scan |
Tyler et al. (1989) | [15O]O2, [15O]CO2, [15O]CO | = | Ipsilateral and contralateral to BAVM | In comparison to control values | |
De Reuck et al. (1989) | [15O]O2 | ↓ | Behind/distant from BAVM | ||
Kaminaga et al. (1999) | [15O]O2 | ↓ | In territory of the carotid artery, ipsilateral to BAVM | ||
Iwama et al. (2002) | [15O]O2, [15O]CO2, [15O]CO | ↓ | Perifocal to BAVM | Only in BAVM with high flow | |
Kuroda et al. (2004) | [15O]O2 | ↓ | Perifocal to BAVM | ||
CBV | Tyler et al. (1989) | [15O]O2, [15O]CO2, [15O]CO | ↑ | Ipsilateral and contralateral to BAVM | In comparison to control values |
Fink (1992) | [15O]CO2 | ↑ | Perifocal to BAVM | In both high- and low-flow perifocal tissue | |
Iwama et al. (2002) | [15O]O2, [15O]CO2, [15O]CO | ↑ | Perifocal to BAVM | ||
Kuroda et al. (2004) | [15O]O2 | ↑ | Perifocal to BAVM | ||
CMRO2 | Tyler et al. (1989) | [15O]O2, [15O]CO2, [15O]CO | = | Ipsilateral and contralateral to BAVM | In comparison to control values
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|