Physics and Chemistry of Radiation Absorption
In 1895, the German physicist Wilhelm Conrad Röntgen discovered “a new kind of ray,” emitted by a gas discharge tube, that could blacken photographic film contained in light-tight containers. He called these rays “x-rays” in his first announcement in December 1895—the x representing the unknown. In demonstrating the properties of x-rays at a public lecture, Röntgen asked Rudolf Albert von Kölliker, a prominent Swiss professor of anatomy, to put his hand in the beam and so produced the first publicly taken radiograph (Fig. 1.1).
The first medical use of x-rays was reported in the Lancet of January 23, 1896. In this report, x-rays were used to locate a piece of a knife in the backbone of a drunken sailor, who was paralyzed until the fragment was removed following its location. The new technology spread rapidly through Europe and the United States, and the field of diagnostic radiology was born. There is some debate about who first used x-rays therapeutically, but by 1896, Leopold Freund, an Austrian surgeon, demonstrated before the Vienna Medical Society the disappearance of a hairy mole following treatment with x-rays. Antoine Henri Becquerel discovered radioactivity emitted by uranium compounds in 1896, and 2 years later, Pierre and Marie Curie isolated the radioactive elements polonium and radium. Within a few years, radium was used for the treatment of cancer.
The first recorded biologic effect of radiation was due to Becquerel, who inadvertently left a radium container in his vest pocket. He subsequently described the skin erythema that appeared 2 weeks later and the ulceration that developed and that required several weeks to heal. It is said that Pierre Curie repeated this experience in 1901 by deliberately producing a radium “burn” on his own forearm (Fig. 1.2). From these early beginnings, at the turn of the century, the study of radiobiology began.
Radiobiology is the study of the action of ionizing radiations on living things. As such, it inevitably involves a certain amount of radiation physics. The purpose of this chapter is to present, in summary form and with a minimum of mathematics, a listing of the various types of ionizing radiations and a description of the physics and chemistry of the processes by which radiation is absorbed.
▪ TYPES OF IONIZING RADIATIONS
The absorption of energy from radiation in biologic material may lead to excitation or to ionization. The raising of an electron in an atom or molecule to a higher energy level without actual ejection of the electron is called excitation. If the radiation has sufficient energy to eject one or more orbital electrons from the atom or molecule, the process is called ionization, and that radiation is said to be ionizing radiation. The important characteristic of ionizing radiation is the localized release of large amounts of energy. The energy dissipated per ionizing event is about 33 eV, which is more than enough to break a strong chemical bond; for example, the energy associated with a C==C bond is 4.9 eV. For convenience, it is usual to classify ionizing radiations as either electromagnetic or particulate.
Electromagnetic Radiations
Most experiments with biologic systems have involved x- or γ-rays, two forms of electromagnetic radiation. X- and γ-rays do not differ in
nature or in properties; the designation of x- or γ-rays reflects the ways they are produced. X-rays are produced extranuclearly; γ-rays are produced intranuclearly. In practical terms, this means that x-rays are produced in an electrical device that accelerates electrons to high energy and then stops them abruptly in a target usually made of tungsten or gold. Part of the kinetic energy (the energy of motion) of the electrons is converted to x-rays. On the other hand, γ-rays are emitted by radioactive isotopes; they represent excess energy that is given off as the unstable nucleus breaks up and decays in its efforts to reach a stable form. Natural background radiation from rocks in the earth also includes γ-rays. Everything that is stated about x-rays in this chapter applies equally well to γ-rays.
nature or in properties; the designation of x- or γ-rays reflects the ways they are produced. X-rays are produced extranuclearly; γ-rays are produced intranuclearly. In practical terms, this means that x-rays are produced in an electrical device that accelerates electrons to high energy and then stops them abruptly in a target usually made of tungsten or gold. Part of the kinetic energy (the energy of motion) of the electrons is converted to x-rays. On the other hand, γ-rays are emitted by radioactive isotopes; they represent excess energy that is given off as the unstable nucleus breaks up and decays in its efforts to reach a stable form. Natural background radiation from rocks in the earth also includes γ-rays. Everything that is stated about x-rays in this chapter applies equally well to γ-rays.
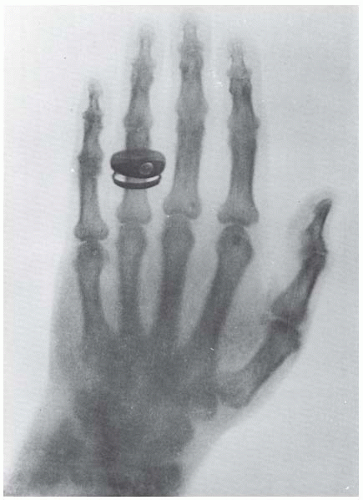 FIGURE 1.1 The first publicly taken radiograph of a living object, taken in January 1896, just a few months after the discovery of x-rays. (Courtesy of Röntgen Museum, Würzburg, Germany.) |
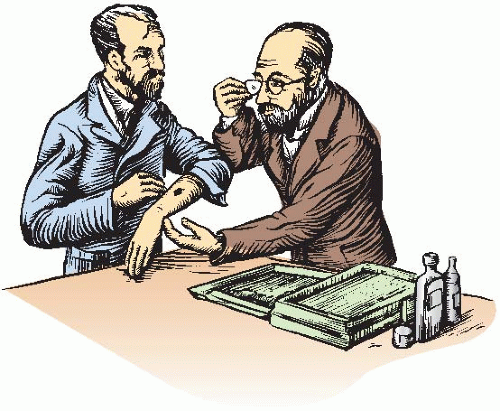 FIGURE 1.2 Based on Becquerel’s earlier observation, Pierre Curie is said to have used a radium tube to produce a radiation ulcer on his arm. He charted its appearance and subsequent healing. |
X-rays may be considered from two different standpoints. First, they may be thought of as waves of electrical and magnetic energy. The magnetic and electrical fields, in planes at right angles to each other, vary with time, so that the wave moves forward in much the same way as ripples move over the surface of a pond if a stone is dropped into the water. The wave moves with a velocity, c, which in a vacuum has a value of 3 × 1010 cm/s. The distance between successive peaks of the wave, λ, is known as the wavelength. The number of waves passing a fixed point per second is the frequency, v. The product of frequency times wavelength gives the velocity of the wave; that is, λv = c.
A helpful, if trivial, analogy is to liken the wavelength to the length of a person’s stride when walking; the number of strides per minute is the frequency. The product of the length of stride multiplied by the number of strides per minute gives the speed or velocity of the walker.
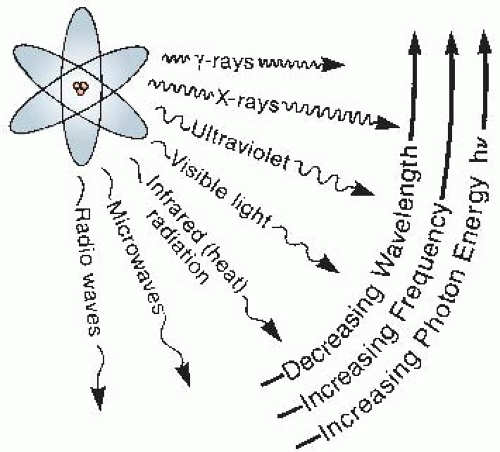
Like x-rays, radio waves, radar, radiant heat, and visible light are forms of electromagnetic radiation. They all have the same velocity, c, but they have different wavelengths and, therefore, different frequencies. To extend the previous analogy, different radiations may be likened to a group of people, some are tall, some are short, all walking together at the same speed. The tall walkers take long measured strides but make few strides per minute; to keep up, the short walkers compensate for the shortness of their strides by increasing the frequencies of their strides. A radio wave may have a distance between successive peaks (i.e., wavelength) of 300 m; for a wave of visible light, the corresponding distance is about 500 thousandths of a centimeter (5 × 10-5 cm). The wavelength for x-rays may be 100 millionths of a centimeter (10-8 cm). X- and γ-rays, then, occupy the short-wavelength end of the electromagnetic spectrum (Fig. 1.3).
Second, x-rays may be thought of as streams of photons, or “packets” of energy. Each energy packet contains an amount of energy equal to hv, where h is known as Planck’s constant and v is the frequency. If a radiation has a long wavelength, it has a small frequency, and so, the energy per photon is small. Conversely, if a given radiation has a short wavelength, the frequency is large and the energy per photon is large. There is a simple numeric relationship between the photon energy (in kiloelectron volts*) and the wavelength (in angstroms†):
For example, x-rays with wavelengths of 0.1 Å correspond to a photon energy of 124 keV.
The concept of x-rays being composed of photons is very important in radiobiology. If x-rays are absorbed in living material, energy is deposited in the tissues and cells. This energy is deposited unevenly in discrete packets. The energy in a beam of x-rays is quantized into large individual packets, each of which is big enough to break a chemical bond and initiate the chain of events that culminates in a biologic change. The critical difference between nonionizing and ionizing radiations is the size of the individual packets of energy, not the total energy involved. A simple calculation illustrates this point. It is shown in Chapter 8 that a total body dose of about 4 Gy‡ of x-rays given to a human is lethal in about half of the individuals exposed. This dose represents absorption of energy of only about 67 cal, assuming the person to be a “standard man” weighing 70 kg. The smallness of the amount of energy involved can be illustrated in many ways. Converted to heat, it would represent a temperature rise of 0.002° C, which would do no harm at all; the same amount of energy in the form of heat is absorbed in drinking one sip of warm coffee. Alternatively, the energy inherent in a lethal dose of x-rays may be compared with mechanical energy or work. It would correspond to the work done in lifting a person about 16 in. from the ground (Fig. 1.4).
Energy in the form of heat or mechanical energy is absorbed uniformly and evenly, and much greater quantities of energy in these forms are required to produce damage in living things. The potency of x-rays, then, is a function not
so much of the total energy absorbed as of the size of the individual energy packets. In their biologic effects, electromagnetic radiations are usually considered ionizing if they have a photon energy in excess of 124 eV, which corresponds to a wavelength shorter than about 10-6 cm.
so much of the total energy absorbed as of the size of the individual energy packets. In their biologic effects, electromagnetic radiations are usually considered ionizing if they have a photon energy in excess of 124 eV, which corresponds to a wavelength shorter than about 10-6 cm.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree