CHAPTER 20 Physics and Instrumentation of Cardiac Single Photon Emission Computed Tomography
Successful performance and interpretation of cardiac single photon emission computed tomography (SPECT) studies relies on a basic understanding of the physics and instrumentation that allows for the generation of the images. The first practical system for the in vivo imaging of radionuclides was developed in the late 1950s by Anger and became commercially available in 1962.1 Despite more than 45 years of use and technical advances, the basic physics and design principles used today for cardiac SPECT imaging are remarkably similar to Anger’s initial concept.
PHYSICS AND SPECT IMAGING
Basic Atomic Structure
The classically described structure of an atom is known as the Bohr atom. It depicts a set of electrons orbiting the nucleus in stable electron shells (K, L, M, N) (Fig. 20-1). Each of the shells represents an energy state, with the innermost shell (K) associated with the greatest potential energy. Each electron’s energy state is defined further by a discrete set of four quantum numbers per electron. The Pauli exclusion principle states that no two electrons in the same atom can have an identical set of quantum numbers. By definition, a stable atom exists in its lowest possible energy state. When the energy state of an electron is increased (i.e., moved to a higher energy orbital shell or via absorption of external energy), the electron emits energy spontaneously as it returns to its lower, more stable energy state. This emission of energy by unstable electrons provides one mechanism for radionuclide imaging and is described in detail in the next section.
Electrons orbit a nucleus composed of a dense conglomerate of protons and neutrons that are bound together by a network of so-called strong nuclear forces. A proton contains a charge of 1.6 × 10−19 coulombs, which is the exact opposite charge of an electron. Neutrons are particles of slightly greater mass than protons, but are uncharged. The number of protons present in its nucleus, or Z number, defines each element, whereas the mass number, or A, represents the number of protons plus the number of neutrons. Specific nomenclature defines the relationship between the number of protons and neutrons in a nucleus (Table 20-1). The ratio of protons to neutrons defines the stability of a particular nucleus (Fig. 20-2); the transformation of an unstable nucleus to a more stable state is responsible for many of the radioactive emissions used in nuclear imaging.
TABLE 20-1 Nuclear Nomenclature
| Name | Symbol | Description |
|---|---|---|
| Z number | ZX | Number of protons |
| A number | AX | Mass number; number of neutrons + protons |
| N number | N | Number of neutrons |
| Nuclide | Common elementary characteristics | |
| Isomer | m | Equal protons and neutrons, different energy state (e.g., Tc 99m) |
| Isotope | Same number of protons; Z1 = Z2 | |
| Isotone | Same number of neutrons; N1 = N2 | |
| Isobar | Same mass number; A1 = A2 |
Radioactivity: Electron Transition, Unstable Nuclei, and Radioactive Decay
Two types of ionization electron transition can occur. In heavy elements (elements with a high number of protons, or Z number), a characteristic x-ray is produced when an electron from an outer shell fills an inner shell vacancy (Fig. 20-3). The energy difference between two electron shells is characteristic of the element, given the different Z number and nuclear charge that define that element. The energy released as the characteristic x-ray represents the difference in potential energy between the outer shell electron and its new quantum state in the inner shell. The movement of an electron from an outer shell to an inner shell produces an x-ray with a characteristic energy, which can be described as a K, L, M, or N x-ray, and the energy of a characteristic x-ray from a particular element can be predicted based on the subatomic structure of the element. An example of a characteristic x-ray emission is the 68- to 70-keV mercury characteristic x-ray that is emitted from thallium 201.
Another mechanism of electron transition that leads to energy emission occurs when an outer shell electron fills an inner shell vacancy, but transfers the energy difference to an outer shell orbital electron (rather than emitting a characteristic x-ray), leading to the emission of an Auger electron (see Fig. 20-3). Because the release of an Auger electron leads to an orbital vacancy, a cascade of subsequent characteristic x-rays or further Auger electrons can occur after the initial emission. Auger electrons are usually produced when orbital electron vacancies are filled in elements of low Z number.
Radioactive decay can involve particulate and nonparticulate emissions. Different types of radioactive decay are described in Table 20-2 and include alpha particle emission, beta particle emission, positron emission, electron capture, gamma ray emission, and internal conversion.
Beta particle emission occurs when a nucleus is unstable because of an elevated neutron/proton ratio (Fig. 20-4). When this occurs, a neutron is converted to a proton with the emission of an electron (β−) and an antineutrino (υ−). Because of the ejection of the electron and antineutrino from the nucleus, the daughter has an atomic number that is one greater than the parent. This decreases the instability generated by the elevated number of neutrons.
In contrast to beta particle (β−) emission, when a nucleus is unstable because of an increased number of protons, radioactive decay can occur through positron emission (β+) or electron capture (see Fig. 20-4). A positron, which is effectively an electron with a positive charge, is emitted during times of proton excess with the simultaneous generation of a neutron. When a positron is emitted from the nucleus, it quickly encounters an electron in the environment, leading to the annihilation of both particles. This annihilation event converts all of the mass of the two particles into energy, with the subsequent generation of two photons of equal energy that travel at 180 degrees to each other. Because the total energy generated by the annihilation reaction is 1.02 MeV, each of the photons emitted from the reaction carries an energy of 511 keV. The high energy of these photons and their simultaneous generation allowing for coincidence detection underlie the mechanisms for positron emission tomography (PET).
A separate process by which a proton-rich nucleus can obtain nuclear stability is through electron capture (see Fig. 20-4). In electron capture, an inner shell electron combines with a nuclear proton to form a neutron, creating a more stable nucleus. An outer shell electron fills the vacant inner shell with the subsequent generation of characteristic x-rays or Auger electrons. Positron emission and electron capture are competitive processes, with β+ emission occurring more frequently in “lighter” elements, and electron capture occurring in “heavier” elements. The characteristic x-rays that are produced during thallium 201 decay (described previously) are produced through electron capture.
Gamma ray emission occurs during nuclear transformation when the process of radioactive decay does not completely dissipate the energy required for the atom to reach its most stable state. Gamma rays are a form of electromagnetic radiation with variable energy without mass or charge (i.e., a photon). Gamma rays carry off the excess nuclear energy through the process of isomeric transition. Isomeric transition occurs when a metastable nucleus is present from a prior radioactive decay. This can commonly occur after β− decay, but can also occur as a consequence of internal conversion (see Fig. 20-4), where an unstable nucleus transfers its energy to an inner shell (K or L) electron, leading to its expulsion as a conversion electron. An outer shell electron, releasing energy via a characteristic x-ray or Auger electron, fills the subsequent electron vacancy. The ability of gamma rays to penetrate tissue (and be used as an imaging tool) depends on their energy. An example of isomeric transition is the gamma photon emitted from the decay of the metastable Tc 99m nucleus.
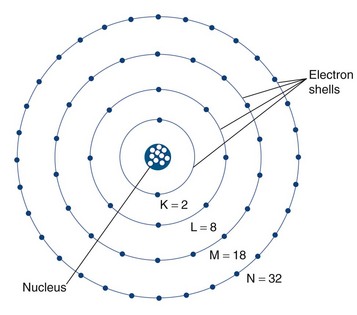
 FIGURE 20-1
FIGURE 20-1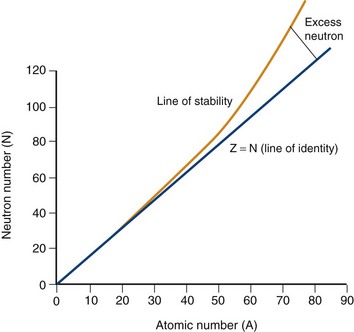
 FIGURE 20-2
FIGURE 20-2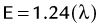
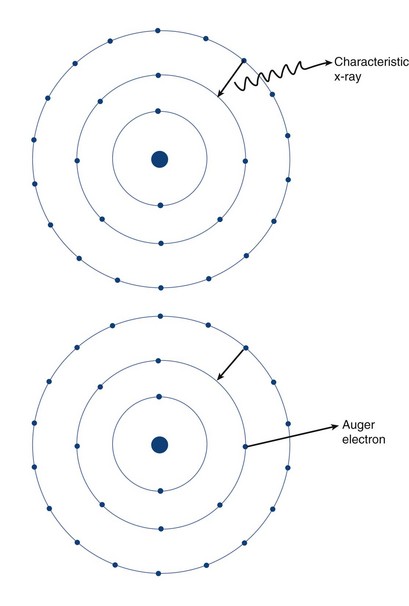
 FIGURE 20-3
FIGURE 20-3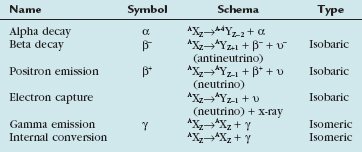
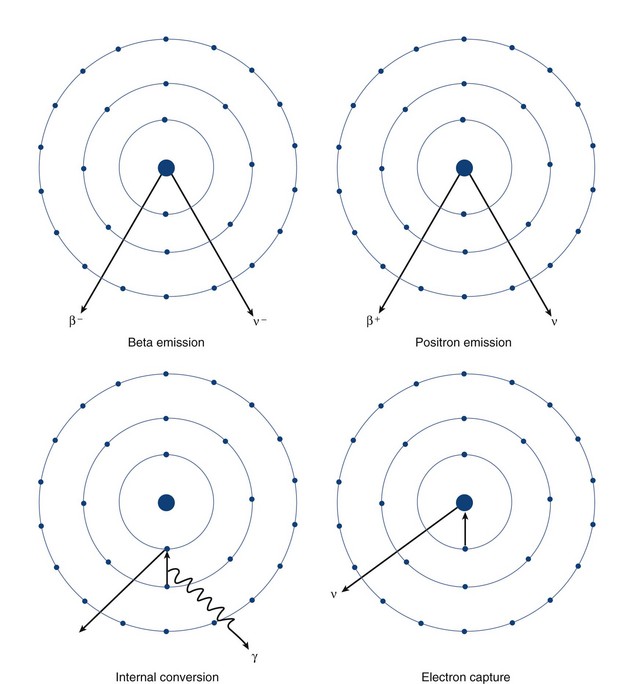
 FIGURE 20-4
FIGURE 20-4


