On plain radiographs, infiltrative diseases usually are classified according to the pattern of abnormality they produce. There are six basic patterns:
Air-space or Alveolar Consolidation
The terms
air-space consolidation and
alveolar consolidation refer to diseases associated with pathologic filling of alveoli (i.e., replacement of alveolar air) as the predominant abnormality. As discussed in detail in
Chapter 2, radiographic abnormalities indicating the presence of air-space or alveolar disease include (1) confluent or homogeneous opacities obscuring vessels, (2) air bronchograms, (3) ill-defined or fluffy opacities, (4) air alveolograms, (5) “acinar” or air-space nodules, (6) preserved lung volume, and (7) a tendency for opacities to extend to pleural surfaces.
The differential diagnosis of diffuse air-space consolidation is reviewed in detail in
Chapter 2 and
Table 2-1. Alveolar filling may be caused by
Water (e.g., pulmonary edema)
Blood (e.g., pulmonary hemorrhage)
Pus (e.g., pneumonia)
Cells (e.g., bronchioloalveolar carcinoma, lymphoma, eosinophilic pneumonia, organizing pneumonia (also known as bronchiolitis obliterans or BOOP), hypersensitivity pneumonitis, interstitial pneumonia)
Other substances (e.g., lipoprotein in alveolar proteinosis, lipid in lipoid pneumonia)
Linear or Septal Pattern
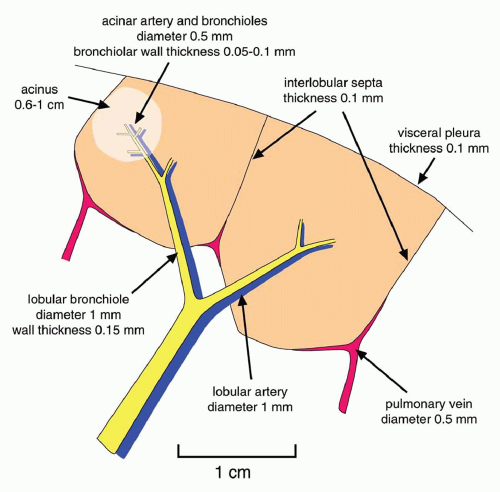
A linear pattern is defined by the presence of Kerley’s A or B lines. Kerley’s A and B lines result from thickening of interlobular septa; this pattern also may be referred to as
septal (
Table 10-1).
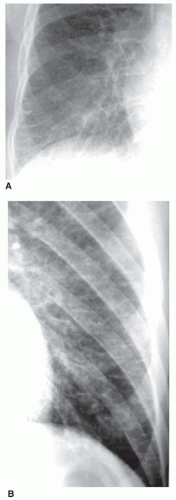
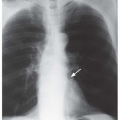
Kerley B lines are most common (
Fig. 10-1). They are horizontal lines, 1 to 2 cm in length. They are seen in contact with the pleural surface and are best seen laterally at the costophrenic angles. The characteristic appearance of Kerley B lines results from the consistent size and regular organization of pulmonary lobules at the lung bases.
Kerley A lines are seen less often. They are oblique in orientation, several centimeters in length, and are located within the central or parahilar lung. Kerley A lines (
Fig. 10-2) also represent thickened septa, but their appearance is different from that of B lines because of the different arrangement of pulmonary lobules in the parahilar lungs.
Kerley C lines are seen at the lung bases and represent interlobular septa en face rather than in profile. They result in a nonspecific, reticular pattern and are unimportant in diagnosis, because B lines invariably are visible as well.
Peribronchial cuffing results from thickening of the peribronchovascular interstitium and also contributes to a linear pattern. This abnormality is seen as thickening of end-on bronchi or as lines radiating outward from the hila. To some extent, peribronchial cuffing contributes to the appearance of Kerley A lines on chest radiographs.
Well-defined and easily recognized Kerley’s lines are indicative of interstitial lung disease and have a limited differential diagnosis. They are typical of interstitial pulmonary edema and lymphangitic spread of carcinoma. Edema usually is symmetrical. Lymphangitic spread often is asymmetrical; asymmetrical Kerley’s lines strongly suggest lymphangitic spread of carcinoma.
Although consolidation may be seen with any type of pulmonary edema (e.g., hydrostatic, increased permeability with or without diffuse alveolar damage, or mixed), a septal pattern more often represents hydrostatic edema or increased permeability edema occurring in the absence of diffuse alveolar damage (see
Chapter 11).
A septal pattern also can result from chronic or recurrent pulmonary hemorrhage and hemosiderosis.
Pulmonary fibrosis sometimes can result in well-defined Kerley’s lines, but a reticular pattern is more typical. Kerley’s
lines in patients with fibrosis are seen most commonly in patients with sarcoidosis.
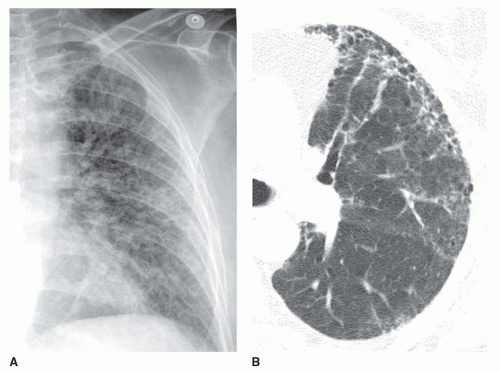
Reticular Pattern
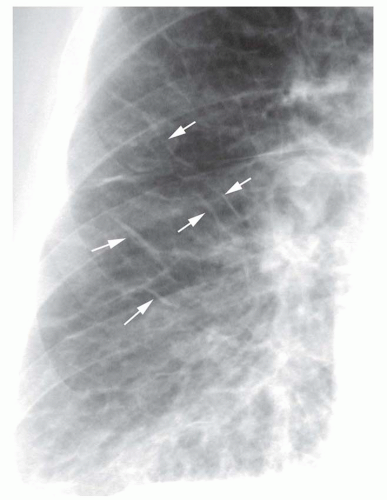
“Reticular” means “netlike,” which is an excellent description of the appearance of this pattern. A reticular pattern is characterized by multiple intersecting lines, often irregular in appearance, outlining round or irregular spaces (
Fig. 10-3). Although a few Kerley lines may be visible, they do not predominate (if they did, the pattern would be linear). A reticular pattern indicates the presence of interstitial lung disease (
Table 10-2).
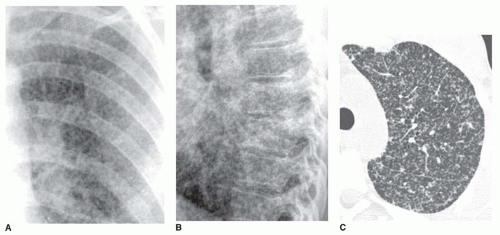
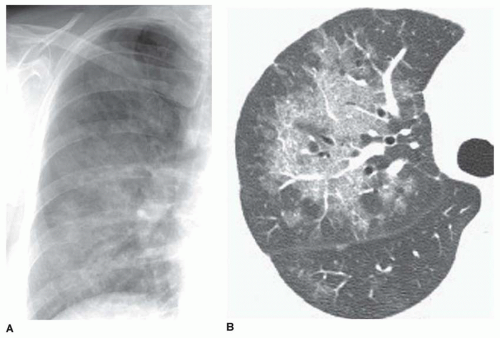
The reticular pattern has been subdivided into three subpatterns, based on the size of the “spaces” surrounded by lines: a fine pattern (spaces smaller than 3 mm;
Fig. 10-4), a medium pattern (spaces 3 to 10 mm;
Fig. 10-5), and coarse pattern (spaces larger than 10 mm;
Fig. 10-6). It should be kept in mind, however, that the size of the spaces visible on chest radiograph do not necessarily reflect the presence or size of the spaces present pathologically. Superimposition of the reticular opacities often confuses the picture; in the presence of extensive reticulation, the outlined spaces usually appear smaller than they really are (see
Fig. 10-4). Medium or coarse patterns are the most common and the most easily seen on chest radiographs.
A medium pattern is typical of patients with pulmonary fibrosis and “honeycombing”; the reticulation often appears to have a peripheral, posterior, and lower lobe predominance (see
Figs. 10-3 and
10-5). The abnormality often is best seen on the lateral view, just above the diaphragm, in the posterior costophrenic angles. Because of lung fibrosis, lung volumes usually appear reduced.
Honeycombing with a medium reticular pattern usually indicates the presence of usual interstitial pneumonia (UIP). UIP can be seen in a variety of conditions; however, over 90% of cases of honeycombing result from a small group of diseases, including idiopathic pulmonary fibrosis (IPF), collagen-vascular disease, drug-related fibrosis, asbestosis, end-stage hypersensitivity pneumonitis, or end-stage sarcoidosis. Nonspecific interstitial pneumonia (NSIP) less commonly results in honeycombing; it often is associated with collagen-vascular disease. Honeycombing also may result from radiation lung fibrosis, as an end-stage of acute respiratory distress syndrome, and other entities. Lung volumes are characteristically reduced in the presence of honeycombing.
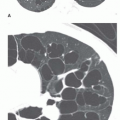
Some cystic lung diseases (e.g., Langerhans’ cell histiocytosis, lymphangiomyomatosis [LAM]) result in a reticular pattern because of superimposition of the walls of cysts ranging in size from several millimeters to several centimeters in diameter (see
Fig. 10-6). Depending on the size of the cysts, the pattern may be fine, medium, or coarse. This appearance can mimic honeycombing, but significant lung fibrosis is absent, and lung volumes are not reduced. In many such patients, lung volumes appear increased. An upper lobe predominance may be seen rather than predominance at the lung bases, depending on the responsible disease.
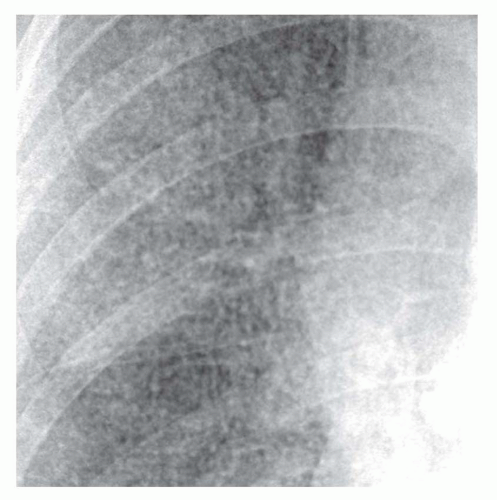
A fine reticular pattern may indicate fine lung fibrosis or lung infiltration by a variety of processes (see
Fig. 10-4). This pattern is less common, more difficult to see, and less specific.
Nodular Pattern
Innumerable small nodules, ranging from a few millimeters to 1 cm in diameter, may indicate interstitial or air-space disease. The differential diagnosis of multiple larger nodules and masses is reviewed in
Chapter 9.
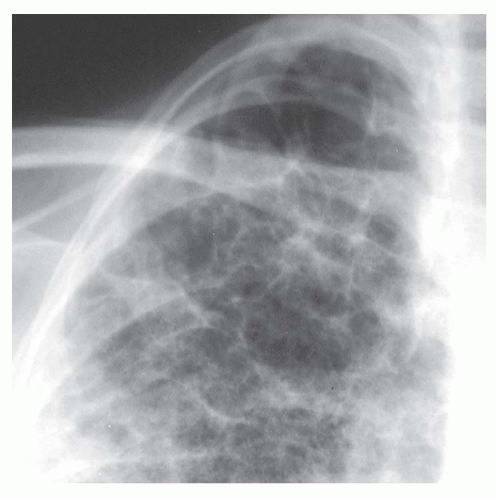
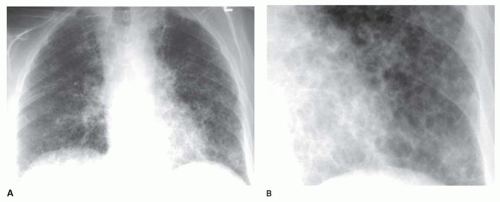
Interstitial nodules usually are sharply marginated, despite being very small (
Figs. 10-7 and
10-8). Air-space disease also may result in nodules (air-space or acinar nodules), typically 5 to 10 mm in diameter and poorly marginated. The term
miliary pattern describes the presence of diffuse or widespread, well-defined nodules, 2 mm or less in diameter (see
Figs. 10-7 and
10-8). Miliary nodules usually are interstitial.
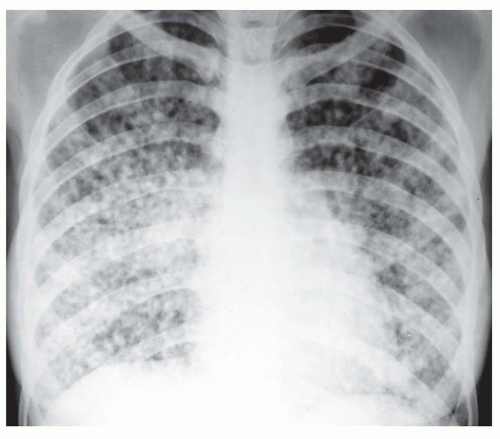
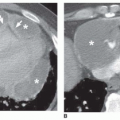
Nearly all patients with nodules that are 5 mm or less in size, either well defined or ill defined, have a predominant interstitial abnormality; many will have metastases (
Fig. 10-9) or a granulomatous disease (see
Figs. 10-7 and
10-8;
Table 10-3). Granulomatous diseases that may produce this appearance include infections (e.g., miliary
tuberculosis and fungus), noninfectious granulomatous diseases (e.g., sarcoid, histiocytosis, hypersensitivity pneumonitis), and some pneumoconioses (primarily silicosis and coal worker’s pneumoconiosis [CWP]). Metastases tend to have a basal predominance because of greater blood flow to the bases (see
Fig. 10-9); the granulomatous diseases and pneumoconioses, for a variety of reasons, often have an upper lobe predominance.
Nodules measuring 5 to 10 mm in diameter may be seen in these same diseases but are more typical of infection, particularly endobronchial spread of infection or bronchopneumonia. Common causes include tuberculosis (
Fig. 10-10) and other mycobacterial, bacterial, viral infections such as cytomegalovirus or varicella, and
Pneumocystis infection in patients with AIDS. Other causes of air-space consolidation also may result in ill-defined nodules. Diffuse bronchioloalveolar carcinoma often results in this appearance.
Reticulonodular Pattern
The term reticulonodular, indicating a perceived combination of lines and dots, is used commonly by radiologists but is of limited value in diagnosis. Reticulonodular opacities observed on plain radiographs often are artifactual, resulting from the superimposition of mostly lines or mostly nodules. Thus, it is generally a good idea, if a reticulonodular pattern is detected on plain radiographs, to decide whether a reticular or nodular pattern predominates and use that finding for differential diagnosis. Cases actually characterized histologically by a combination of reticular and nodular opacities are relatively uncommon but include sarcoidosis, lymphangitic spread of tumor, and diffuse amyloidosis.
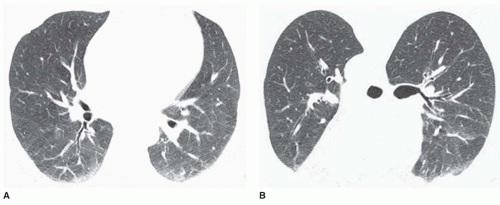
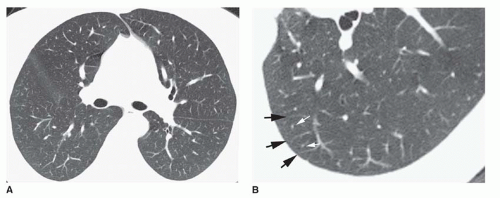
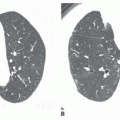
Ground-glass Opacity
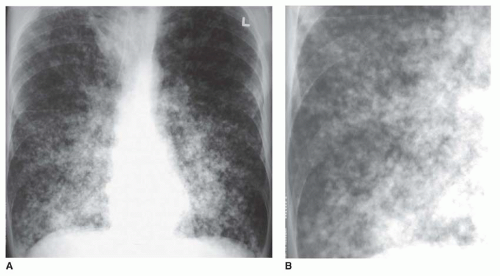
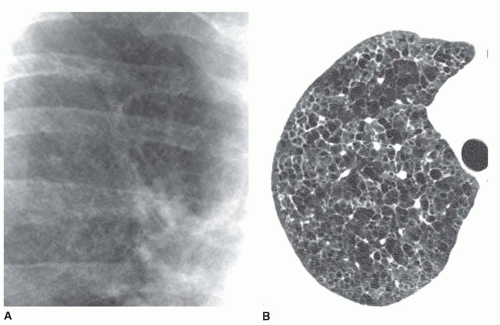
Ground-glass opacity represents an increase in lung density without the presence of frank consolidation (
Fig. 10-11A). A slight fuzziness of pulmonary vessels usually is visible,
but this abnormality can be quite subtle and difficult to diagnose with certainty. It is a nonspecific pattern (see the following section on HRCT) and can be seen in the presence of either air-space disease or interstitial disease. Because it is nonspecific, its differential diagnosis is very long. It may be seen with edema, hemorrhage, infections, and a wide variety of different DILDs. When visible on a chest film, it is best evaluated by assessment of the history and clinical presentation in patients with acute symptoms or by further imaging (e.g., HRCT) in patients with chronic symptoms (see
Fig. 10-11B).