Ports
Whenever humans have journeyed into remote or hostile regions, the success of the undertaking has depended largely on the procurement and maintenance of vital supply routes. For the mountaineer attempting to summit Everest, it is the Sherpas who carry the supplies necessary for a successful venture. For the physician faced with the challenge of treating complex medical ailments, it is reliable access to the venous system that is necessary for success. Among the first to recognize this was Dudrick who in 1968 reported the use of percutaneous infraclavicular subclavian vein catheters for the administration of hypotonic parenteral nutrition solutions.1 Since then, many modifications to this basic design concept have been made to improve the function and safety of these devices.
The earliest modifications led to the development of tunneled Silastic catheters, first introduced by Broviac et al2 and later modified by Hickman et al.3 These devices improved the overall safety of long-term venous access by reducing the inherent risk of infection. While a significant improvement over their nontunneled predecessors, tunneled catheters possess externalized components and are therefore at moderate risk for catheter dislodgment and sepsis. Totally implantable subcutaneous venous-access devices (SVADs), introduced in 1982, possess no externalized components, and therefore permit unrestricted patient activity, and are more resistant to infection.4 These advantages led to the widespread use of these systems for the delivery of therapeutic agents.
Unfortunately, iatrogenic complications related to device implantation still occur with significant incidence.5 As a result, device design and implantation techniques have continued to evolve. This evolution has led to the use of sophisticated imaging tools and catheter techniques to help guide device implantation. This approach can reduce the incidence of access-related complications significantly and improve implantation success and long-term device function. This chapter focuses on the use of these techniques for SVAD implantation and provides the reader with an overview of the various device designs, implantation indications, and outcomes.
 Port Designs
Port Designs
The port is a rather simple device. Its only function is to provide a subcutaneous doorway into a catheter for the administration or removal of fluid. The catheter is usually placed in a central vein, but this need not always be the case. Catheters can be positioned in the arterial system, thecal sac, pleural space or peritoneal space. Aside from serving as a transcutaneous access point for the implanted catheter, the port is designed to prevent infection and maintain patency of the catheter conduit. The simplest port design is a small, round fluid-tight container with a thick silicone roof. A hole in the side of the container leads to the access catheter. The silicone roof is designed to tolerate penetration by a specialized needle so that access to the inside of the container can be achieved. When the needle is removed, the silicone septum seals the needle tract and restores the device to its impervious condition (Fig. 26–1).
Manufacturers have made many modifications to this basic design. Alterations in container shape and size made the device more versatile and suitable for placement at various anatomic locations. As a result, a wide variety of devices exist, each of which has unique characteristics that make it most suitable for a specific use. The peripheral port, which is a miniaturized version of the aforementioned port design, is an example of a unique device modification (Fig. 26–2). Its small size permits placement in the arm or forearm, usually connected to a catheter inserted into a peripheral vein. Placement at this location avoids the risks associated with accessing central venous structures. Another design modification led to the development of double-lumen devices, which allow simultaneous administration of different therapeutic agents and therefore can accommodate patients with complex access needs.
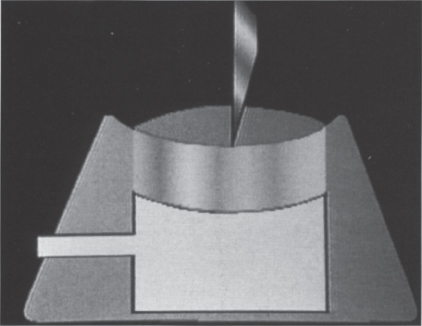
FIGURE 26–1. Basic port design consists of a rigid container with a thick silicone septum.
Each of the previously mentioned modifications, although unique, still consists of a container with a silicone septum. This design has an inherent disadvantage in that it possesses a reservoir. The presence of a reservoir, however small, allows for the accumulation of debris and increases the likelihood of infection and device malfunction. More radical design modifications have focused on this problem. The Vortex Port (Horizon Medical products Inc., Manchester, GA) formerly known as the OmegaPort (Norfolk Medical, Skokie, IL), has a sphere-shaped container with no corners where debris could accumulate (Fig. 26–3). One article reported that 50 consecutive OmegaPorts were free of withdrawal occlusions on follow-up,6 suggesting that the device effectively reduced debris accumulation. This report did not, however, describe the contents of the reservoirs. Other investigators have found this design not to be effective, finding large amounts of particulate matter within the reservoir at explantation.7
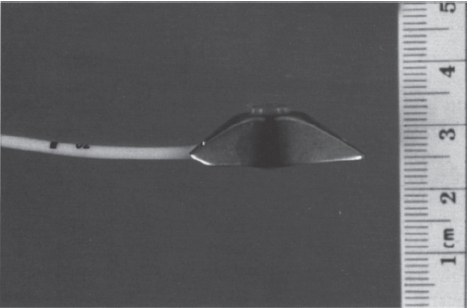
FIGURE 26–2. The reduced size of peripheral ports allows them to be implanted in small body parts (PAS Port, Pharmacia Deltec, St. Paul, MN).
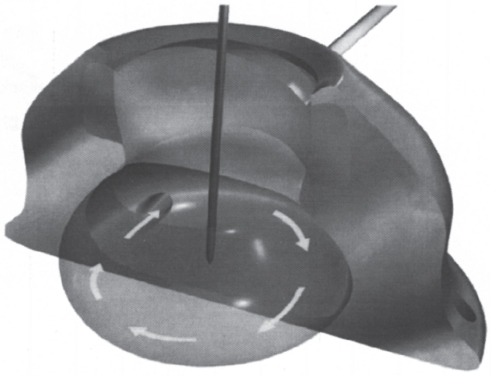
FIGURE 26–3. Diagram of Vortex Port™ (Horizon Medical Products Inc., Manchester, GA) demonstrating the rounded internal reservoir with uniform fluid flow pattern. This container design has no edges that could allow debris accumulation.
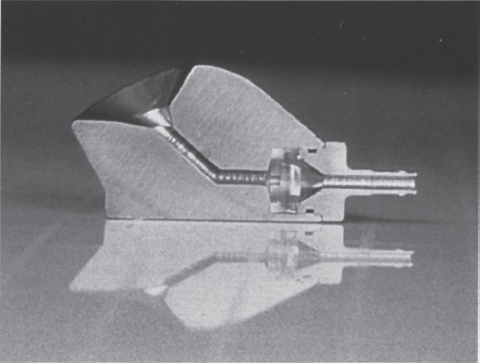
FIGURE 26–4. Cutaway of CathLink Port™ (Bard Access Systems, Salt Lake City, UT) demonstrating channel and silicone septum that IV catheter must traverse to reach the interior of the access catheter.
Other designs have addressed this problem by eliminating the reservoir portion of the device entirely. An example of this is the CathLink device (Bard Access Systems, Salt Lake City, UT). This device is radically different from most other port designs. It is accessed with an intravenous (i.v.) catheter instead of the usual rigid needle. The i.v. catheter is directed through a passage in the device, which contains three silicone septums, penetrates the septum, and actually enters the lumen of the attached catheter conduit (Fig. 26–4). With inline access to the catheter, obstacles to blood flow are eliminated and debris cannot accumulate:
Inline access designs can also permit high blood flow rates with minimal turbulence and hemolysis. As such, this design is ideal for hemodialysis use. Currently, two manufacturers have used this concept to develop implantable devices that can provide the blood flow rates necessary for hemodialysis. The LifeSite (VascA Inc., Tewksbury, MA), and the Dialock (BioLink Corp, Middleboro, MA) are currently under investigational device exemption (IDE) investigation in United States. The early results of these clinical trials appear promising. The LifeSite device (Fig. 26–5), in more than 59 implants with more then 6000 days of access, has demonstrated mean blood flow rates of 388 mL per minute, with a low infection rate. Five devices were removed because of infection, but no devices were removed for skin breakdown, device failure, or thrombosis. Although adequate data for the Dialock device are lacking, similar results have been reported.8,9

FIGURE 26–5. LifeSite™ (VascA Inc., Tewksbury, MA) mechanism. As the access needle enters the port channel it displaces ball bearings laterally. This forces channel downward to release the pinch lock (white area). Once released, needle is in continuity with catheter lumen.
 Indications and Contraindications
Indications and Contraindications
Totally implanted subcutaneous venous access devices have a distinct advantage over other types of access by virtue of the fact that they have no externalized components; however, these devices have their limitations, and the decision to implant an SVAD should be considered carefully. One significant limitation is cost. SVADs costs three to five times more than tunneled access catheters. However, this initial cost difference, over time, becomes less significant when the cost of catheter care is factored. Tunneled catheters require frequent dressing changes and need to be flushed with a heparinized solution daily when not in use. SVADs, when not in use, require no dressing changes and need to be flushed only once a month. After 5 to 6 months, the cost of catheter care for tunneled devices will exceed the higher initial cost of SVAD implantation.10 With continued use, SVADs become less and less expensive and therefore more cost-effective. Thus, it is our recommendation that SVADs implantation be reserved for patients who require access for longer than 5 months.
Under certain circumstances, this recommendation need not be followed strictly. In situations where certain activities are critical to a patient’s lifestyle, it may be prudent to place a totally implantable device. For example, a scuba diving instructor requiring a 6-week course of intravenous antibiotics would benefit from an access device that would allow continuing his or her vocation. Additionally, in some situations, the risk of device dislodgment is so great that an implantable device is preferred and ultimately more cost-effective. This scenario may arise, for example, when treating mentally incompetent patients, hyperactive children, and prison inmates. In prison, hostile interactions between cell mates can reduce the longevity of any access device with externalized components.
Once the decision has been made to place an SVAD, the next important step is to decide what type of device to place, peripheral or central, large or small, single or double. Many of these decisions are based on the preference of the physician and, occasionally, the patient. The decision to choose a single- or a double-lumen device requires careful consideration. The addition of a second lumen comes at an increased risk to the patient. Studies have demonstrated a higher incidence of infection and thrombosis with larger multilumen catheters.11–14 The concept of “two is always better than one” does not apply to venous access. If a single lumen is all that is clinically needed, a double-lumen device should not be implanted.
Bacteremia and disseminated intravascular coagulation should be regarded as absolute contraindications to SVAD implantation.15 Recent but resolved sepsis, neutropenia, and mild coagulopathies are relative contraindications. In these situations, the increased risk of implantation must be weighed against the patient’s need for this type of access. Another relative contraindication is related to device use. If a patient’s clinical condition requires continuous device access, then an SVAD is a poor choice. When an SVAD is accessed, the needle disrupts the skin barrier and serves as a conduit for device contamination. In our experience, the longer the device remains accessed, the greater the risk of infection. Once infection develops, device removal is often necessary. Compared with tunneled catheters, SVAD removal is far more traumatic and complicated, particularly when a local infection prevents immediate closure of the explanting incision. Additionally, although an SVAD is accessed, unrestricted patient activity is lost. If accessed all the time, this device offers no advantages over externalized catheters. It is therefore our recommendation to avoid SVAD implantion if continuous access is required.
 Implantation Technique
Implantation Technique
Implantation Site Selection
When choosing an access site, it is important to be aware of the patient’s previous access and surgical history. Prior radiation exposure or extensive surgical dissection often is associated with central vein occlusions. If possible, these areas should be avoided when placing a new device. Additionally, the patient’s personal needs must be understood before an access site is chosen. Often the patient may have specific preferences as to where an access device should be located. The chest wall may be preferable to the arm in some patients and the opposite in others. If possible, these preferences should be considered before the final access site is chosen.
Implantable devices can gain access to the central venous system from either peripheral or central locations. Whereas peripherally placed SVADs have become more popular over the past decade, centrally placed chest-wall SVADs still constitute a majority of the implanted devices. The subclavian veins, because of their location on the chest wall and the broad familiarity with subclavian access techniques, are the most frequently used access veins. Access into these veins, however, is associated with significant long- and short-term complications.16 Pneumothorax and symptomatic central venous thrombosis are more likely to occur with subclavian vein access,17,18 even when imaging techniques are used to gain access.19,20 Jugular vein access is significantly safer; this access, in the short term, avoids pneumothorax and in the long term reduces the rate of symptomatic central vein thrombosis. Yet internal jugular vein access is still commonly avoided when placing implantable devices. What deters implanting physicians from using this approach is the tortuous course the catheter must take as it comes across the neck down to the chest wall.
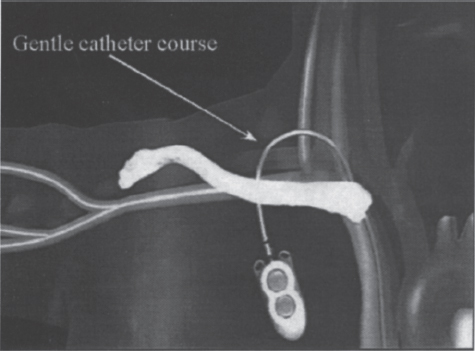
FIGURE 26–6. The low posterior approach allows the catheter to assume a gentle curve as it is tunneled over the clavicle to the chest wall.
A low posterior approach to the internal jugular vein, which is described in detail later in this chapter, solves this particular problem. When the vein is accessed in this fashion, the catheter can assume a gentle curve as it courses through its subcutaneous tunnel (Fig. 26–6). Using this access approach, we implanted 164 SVADs and compared the outcomes with those of our earlier experience with 152 implantable devices that used subclavian vein access. The access catheter diameter, pathology mix, and implantation time were similar in both groups. Insertion was technically easier with low posterior internal jugular vein approach, and device function was better in this group. Pneumothorax and symptomatic central vein thrombosis occurred in the subclavian access group with an incidence of 1.9% and 4.5%, respectively. None of these complications occurred in the internal jugular vein access group. The results of this study substantiated the advantages of this approach, and it remains our preferred technique.
In patients with occluded internal jugular and subclavian veins, alternate approaches are necessary. Placement of a chronic catheter in the common femoral vein is an option; however, this approach is associated with a high incidence of lower extremity deep-vein thrombosis (DVT) and an increase infection rate.21 With good radiologic guidance, access into the inferior vena cava can be obtained through a translumbar or transhepatic approach, thereby providing an alternate route for chronic circulatory access.22,23
Site Preparation
Ultrasound Survey
Before the skin is prepared for the surgical procedure, it is important to know whether the intended access vein is patent. It is a waste of time and effort if it is discovered that the vein is not patent after the site has been prepared. Knowledge of the patient’s prior access history and a quick physical examination can help to determine the patency of central veins. Occasionally, the patient may know which veins are patent and which veins are occluded. If not, physical findings, such as large venous collaterals on the chest wall and shoulder, can suggest the presence of an occluded vein. Although these techniques are helpful, the best way to determine whether the vein is patent is to perform a quick ultrasound examination. If the intended access vein is found to be easily compressible and can be followed down toward the chest, then access difficulties are unlikely.
Skin Preparation
It is impossible to sterilize the skin completely. Therefore, the goal in preparing a surgical site is to remove the transient and pathogenic bacteria and to decrease the resident flora to the lowest possible level, which can best be accomplished through the combined effects of a mechanical scrub and an effective antiseptic agent.24 Prior to preparing the skin, hair removal may be necessary. Shaving with a razor can traumatize the skin surface and enhance bacterial growth, but this only occurs if shaving is performed several hours before the procedure.25,26 If hair removal is absolutely necessary because it would interfere with the surgery, mechanical clipping is preferable to shaving.24 If mechanical clippers are unavailable, shaving should be done just prior to the surgery using an antiseptic scrub as a lubricant.
The antiseptic preparation of the skin should be a two-step process. The first step is a mechanical scrub using chlorhexidine (Hibiclens, Stuart Pharmaceuticals, Wilmington, DE). Chlorhexidine is effective against a wide range of gram-positive and gram-negative bacteria. Many studies have shown chlorhexidine to be superior to iodophors and alcohol as an antiseptic agent.27–29 The second step involves painting an antiseptic solution over the skin, usually, iodophor compounds, such as povidone–iodine. These compounds are effective against a wide range of bacteria.30 Once applied, the iodophor compound can be allowed to air dry on the skin, but several studies have shown this not to be essential.31,32 If patients are sensitive to iodine based compounds, isopropyl alcohol can be used instead.
Prophylactic Antibiotics
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree