CHAPTER 103 Postendograft Imaging of the Abdominal Aorta and Iliac Arteries
The primary complication of abdominal aortic aneurysm (AAA) is acute rupture, a frequently lethal event.1 As a rule, even large abdominal aortic aneurysms are asymptomatic until rupture occurs.2,3 In the United States, a ruptured abdominal aortic aneurysm is the 10th leading cause of death in patients older than 55 years. Risk of rupture is directly related to aneurysmal size, with a 5% to 10% annual risk of rupture for AAAs measuring between 5 and 6 cm. There is controversy regarding the exact size at which an aneurysm should be repaired. Surgical repair has been advocated for AAAs reaching 5.0 to 5.5 cm in size by several authors; however, open surgical repair is a major operation, with an overall operative mortality ranging between 4% and 8.4%.3,4 Endovascular repair of abdominal aortic aneurysms with the use of stent grafts has emerged as a new imaging-guided procedure (Fig. 103-1). The use of stent grafts for repair of AAA in humans was first described in 1991 by Parodi and colleagues,5 who constructed devices from Palmaz stents and a standard woven polyethylene terephthalate surgical graft material.
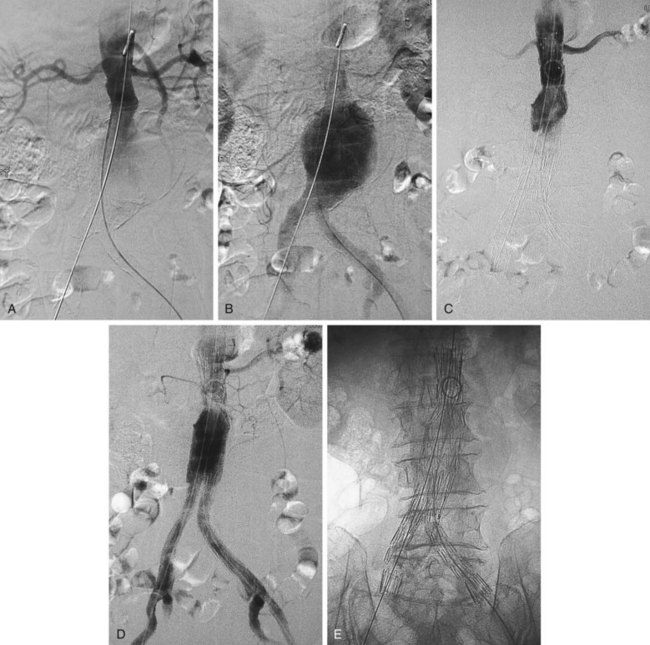
 FIGURE 103-1 Angiographic appearance of an abdominal aortic aneurysm before (A, B) and after (C, D, E) stent graft placement.
FIGURE 103-1 Angiographic appearance of an abdominal aortic aneurysm before (A, B) and after (C, D, E) stent graft placement.
Similarly, the natural course of an iliac artery aneurysm (IAA) consists of progressive expansion with eventual rupture. Involvement of iliac arteries is seen in 10% to 20% of patients with AAAs.6 IAA is defined as enlargement of the iliac artery to a diameter of more than 1.5 cm. The natural course of an IAA consists of progressive expansion with eventual rupture, and the risk of rupture increases with aneurysm size.7 Surgical repair should be recommended for isolated IAAs larger than 3.0 to 3.5 cm in diameter.8,9 A variety of minimally invasive therapeutic options have now become available (e.g., coil embolization, stent graft placement; Fig. 103-2), and choosing an appropriate option is essential for achieving optimal long-term results and reducing potential complications.10
The ultimate goal of endovascular repair of AAA and IAA with a stent graft is the same as for surgical repair—that is, depressurization and exclusion of the aneurysm sac from the circulation to prevent rupture. The dominant limiting factor in patient selection is the stent graft itself.11,12 Each device has specific and relatively restrictive requirements with regard to the diameter, length, and angulation of the proximal and distal attachment sites, and to the ability of the iliofemoral arteries to accommodate the stent graft delivery systems. A certain length of nonaneurysmal infrarenal aortic length, depending on the device used, must be present for proper placement of the proximal attachment of the stent graft. Aortic neck angles more than 45 degrees may pose problems with implantation and may stress the device. Trapezoidal, conical, or thrombus-filled aortic necks may cause device instability. Additionally, the iliofemoral vessels must have a certain (device-dependent) caliber and straightness. Patients who do not fit the device cannot be treated.13
POSTOPERATIVE ASSESSMENT
Endograft placement is relatively new, and studies regarding its long term efficacy are still being performed to define its clinical role better. In the Endovascular Aneurysm Repair (EVAR) trial 1,14 the 30-day mortality rate for endovascular repair was 1.7% and the corresponding rate for open repair was 4.7% (P < .001). At 4 years, the aneurysm-related mortality rate in the group of patients who underwent endovascular repair was half that of the group of patients who underwent open repair (P = .04), but there was no significant difference in mortality from any cause (26% for endovascular repair and 29% for open repair). However, a higher percentage of patients (20%) who underwent endovascular repair required reinterventions (compared with 6% in the open-repair group). Therefore, although perioperative mortality was higher in patients who underwent open repair, a higher percentage of patients in the endovascular repair group required reinterventions, and there was no significant difference in 4-year mortality rates between the two groups.
In the Dutch Randomized Endovascular Aneurysm Management (DREAM) trial,15 the 30-day mortality rate was significantly lower in patients who had undergone endovascular repair compared with those who had undergone open repair. However, the overall survival rate was not significantly different between the two groups at 2 years. Although aneurysm-related death was more frequent in patients who had undergone open repair, reinterventions were required more often in patients who had undergone endovascular repair. These results corroborate those of the EVAR trial 1. In the EVAR trial 2,16 338 patients with AAA who were not candidates for open repair were randomly assigned to undergo endovascular repair or no intervention. No benefit of endovascular repair was apparent during follow-up. Complications and subsequent interventions were more frequent in the group of patients who underwent endovascular repair. Therefore, the benefit of endovascular repair in patients who are not surgical candidates appears unclear.
Successful endovascular repair of AAA and IAA remains technically challenging despite continued improvement in device design. As noted, additional interventions may be required before or during stent graft placement.13 Primary technical success is defined on an intent to treat basis and requires the following: (1) successful insertion and deployment of the graft without the need for surgical conversion; (2) no perioperative mortality; (3) absence of type I or III endoleak; and (4) freedom from limb obstruction or occlusion, up to 24 hours postoperatively. Clinical success of stent grafting is defined as technical success in conjunction with freedom from aneurysm-related death, type I or III endoleak, graft infection or thrombosis, aneurysm rupture, conversion to open repair, or graft migration during the life of the patient.17
Occlusion of branch vessels arising from the proximal neck, aneurysm sac, or iliac arteries is the most common preprocedural intervention.13,18 This is done to provide appropriate attachment sites or prevent retrograde perfusion of the aneurysm sac after placement of the stent graft. Contralateral common iliac artery occlusion is required when inserting an aortounilateral iliac artery stent graft. In a patient with a common or internal IAA, embolization of the internal iliac artery is required before stent graft is deployed across its origin into the ipsilateral external iliac artery (Fig. 103-3). Interventions such as angioplasty or stent placement may also be required during insertion of a stent graft to accommodate large delivery systems better.13 Angioplasty to dilate conduit artery stenoses should be performed at the time of the stent graft procedure, not before. At times, the main body of the graft may have to be inserted through the contralateral side from the one initially planned if difficulties are encountered during its insertion because of a stenosis (Fig. 103-4). The threshold for stent placement to repair a traumatic dissection (Figs. 103-5 to 103-7) caused by a large delivery system or to buttress the iliac limb of an unsupported stent graft should be low. Aortounilateral iliac artery stent grafts require a surgical femoral to femoral bypass graft to perfuse the pelvis and limb contralateral to the stent graft. A vascular plug to occlude flow through the contralateral common iliac artery should be placed immediately prior to the surgical femoral to femoral bypass.
Successful insertion of an aortic stent graft is achieved in more than 95% of procedures in carefully selected patients and appropriately chosen devices. The most common cause of a failed procedure is the inability to insert a device through diseased or tortuous iliac arteries, especially with devices with large profiles. Misplacement of the stent graft, failure to deploy, or acute and irreversible occlusion (Fig. 103-8) are unusual events but may require urgent open surgical repair. This is termed a surgical conversion and is associated with a higher morbidity than conventional open surgical repair.
Death during a stent graft procedure is very rare. Acute intraprocedural rupture of an AAA during stent graft placement with successful outcome has been reported but is also rare. Complications such as multiorgan system failure, myocardial infarction, bowel infarction, stroke, pulmonary embolism, and peripheral arterial embolism have all been described after stent graft procedures, but most early complications are minor and mainly consist of injuries to access arteries or issues related to groin incisions.13
As noted, complete exclusion of the aneurysm sac is the goal of stent graft placement and the definition of early clinical success.19 Figure 103-9 demonstrates the expected anatomic appearance after successful deployment of a stent graft on CT angiography.
Endoleak
Persistent opacification of the aneurysm sac after insertion of a stent graft is termed an endoleak and is classified by cause and time of occurrence.20 This classification of endoleaks is described in Table 103-1. Most early endoleaks are currently types II and IV. Type I leaks can be minimized by careful patient selection and preprocedural measurements. Tube stent grafts may have a higher rate of type I leak, particularly at the distal attachment site, than bifurcated stent grafts and today are used for selected patients only. Large attachment leaks that result in continued pressurization of the aneurysm sac indicate a failed procedure and may leave the patient at risk for subsequent AAA rupture. Because more than 50% of small endoleaks will resolve spontaneously, their management is usually expectant.13 Although many early endoleaks disappear within 6 months, reappearance of leaks and delayed appearance of new leaks can occur at any time. Stabilization of aneurysm size or even shrinkage and avoidance of secondary endovascular procedures or open surgery are considered measures of long-term success.
TABLE 103-1 Endoleak Classification
| Type of Endoleak | Features |
|---|---|
| I |
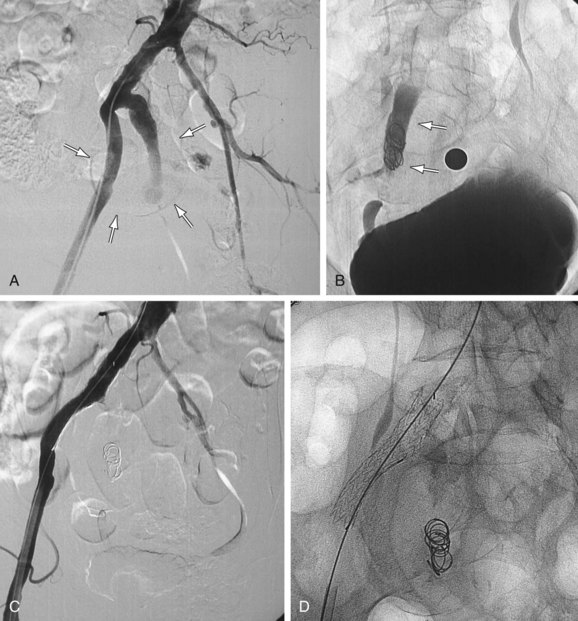
 FIGURE 103-2
FIGURE 103-2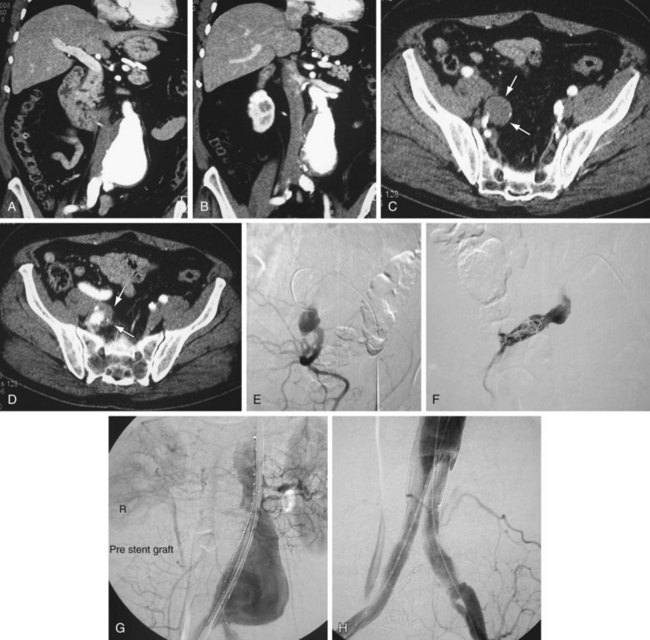
 FIGURE 103-3
FIGURE 103-3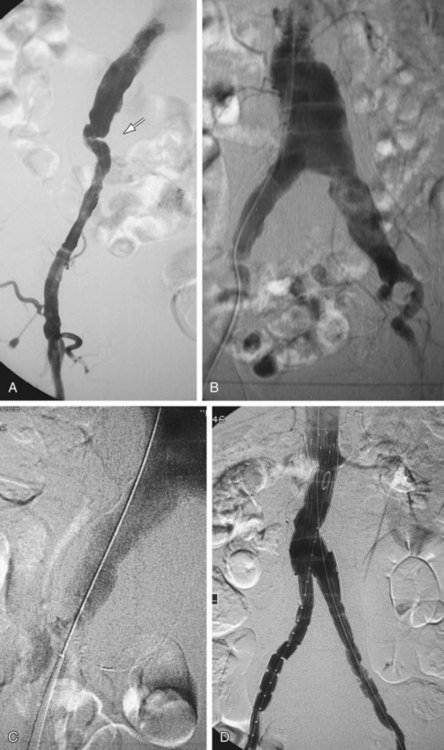
 FIGURE 103-4
FIGURE 103-4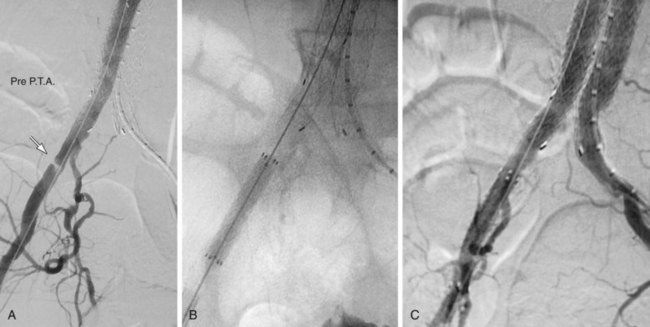
 FIGURE 103-5
FIGURE 103-5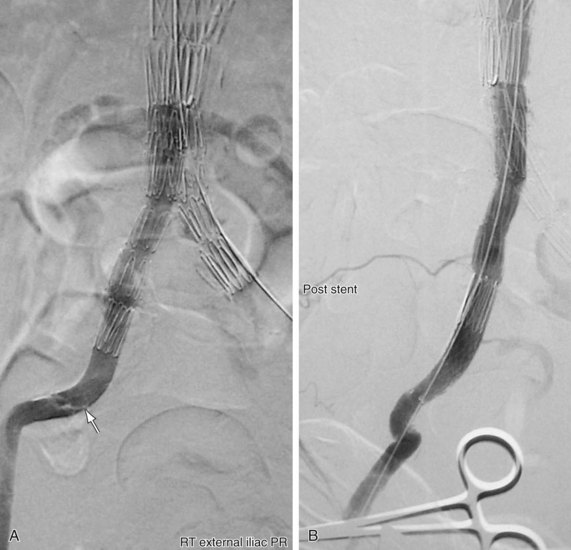
 FIGURE 103-6
FIGURE 103-6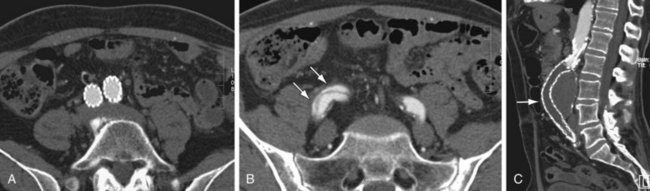
 FIGURE 103-7
FIGURE 103-7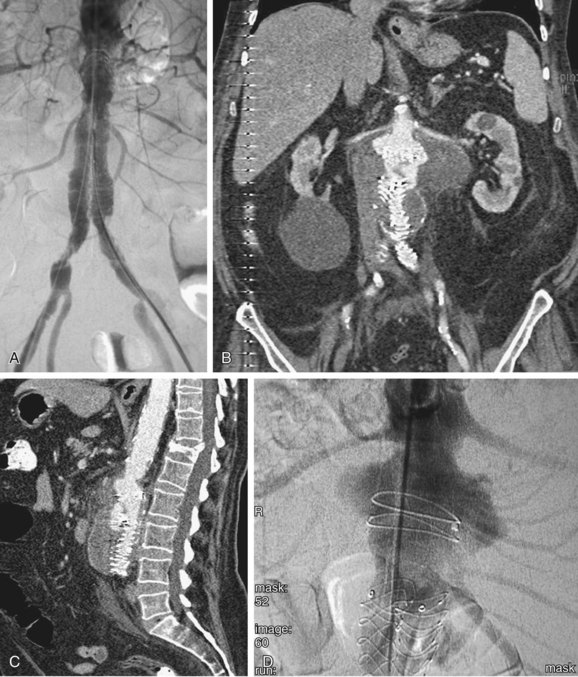
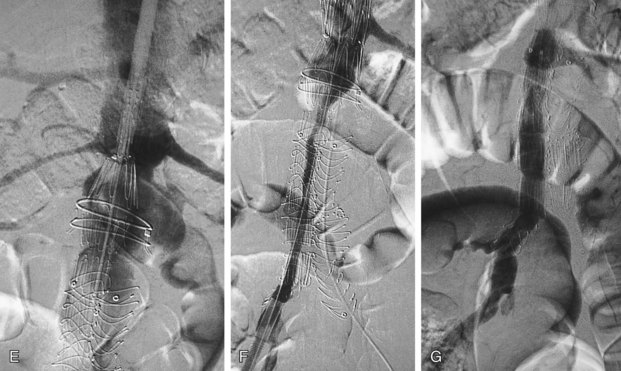
 FIGURE 103-8
FIGURE 103-8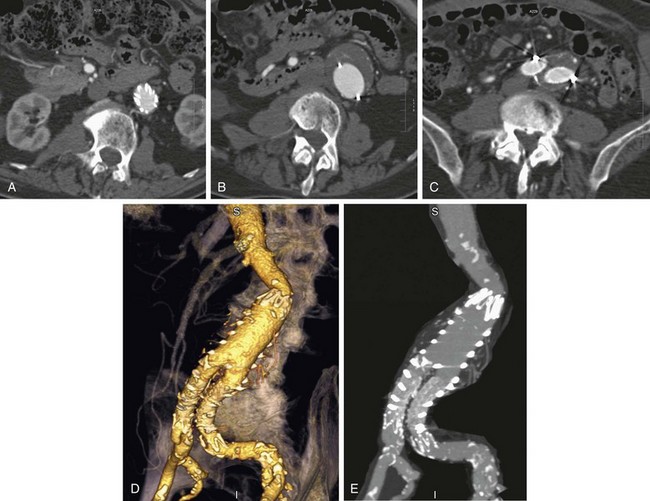
 FIGURE 103-9
FIGURE 103-9


