Postmortem imaging has become an increasingly important part in the examination of deceased fetuses and neonates. This article discusses how to perform magnetic resonance (MR) imaging autopsy, including such technical determinators as field strength, and factors influencing image quality. Also included is a discussion on the indications for MR autopsy, including stillborns and terminations of pregnancy, and natural and unexplained death.
Today postmortem imaging has become an increasingly important part of the examination of deceased fetuses and neonates. For centuries postmortem examination consisted only of a classical autopsy, which is still considered to be the gold standard. The pathologist’s role is to establish the exact cause of death, which is often an important factor in the bereavement process of parents, especially when they are concerned about the correctness of their decisions during pregnancy. However, identification of the reason for fetal and neonatal death serves many more important goals. Autopsy results are also of great value for genetic counseling, not only for assessing the recurrence rate of a specific syndrome but also for implementation of strategies for prevention of unnecessary risks in future pregnancies. Risk estimation for recurrence may, however, be hampered by parental refusal of autopsy because of religious or philosophic beliefs. In this case, one can only rely on prenatal tests for counseling of future pregnancies. Another important issue is the medicolegal aspect, where postmortem confirmation of the prenatal diagnosis or suspected fetal pathology may validate the performed obstetric strategy. This may relieve both parents and physicians of blame. Finally, identification of the exact fetal pathology and cause of death advances medical knowledge by allowing clinicians to monitor and validate therapies, which eventually improves clinical practice and research.
Notwithstanding the acceptance of the undisputable value of autopsy worldwide, autopsy rates are continuously declining. Many studies, including those meta-analysis based, show high clinicopathologic discrepancy rates, varying between 22% and 76%. Additionally, one autopsy study shows a change in recurrence risk estimation or parental counseling in more than 26% of cases. Therefore, it is of utmost importance to understand why autopsy rates are declining and, if this process is inevitable, to explore and develop alternative postmortem diagnostic techniques.
The reason for the decline in autopsies is multifactorial and complex. The impact of the perception and acceptance by the general public, clinicians, and pathologists of autopsies is significant. The low public consent rate is one of the most important reasons for the observed decline. The consent rate seems to depend on religion, ethnic origin, cultural attitude, media portrayal of autopsies, and public perceptions. Religious and ethnic beliefs may strongly oppose autopsy and anatomic dissection. In addition, the religious necessity that the burial takes place within 24 hours may also interfere with or prevent autopsy. As a result, the fetal or neonatal brain has to be examined unfixed, preventing detailed analysis. Newer agents, such as picric acid and formaldehyde, make an adequate overnight fixation and immersion possible, but the technical limitations of removing the watery, unfixed brain from the cranial vault are still tremendous. With regard to the clinician, the decline in autopsy rate is caused by the time-consuming consent process, the assumption that advances in premortem diagnostic techniques diminish the need for autopsy, and that a grieving family is hostile to the idea of autopsy. For some pathologists autopsy is an unpleasant, time-consuming procedure often regarded as secondary to their primary duties, best delegated to a junior trainee. Also, the lack of communication between the clinician and the pathologist further limits the clinical value of autopsy.
Therefore, there is a continuous need to improve the awareness of the clinical value of autopsy not only for clinicians and pathologists, but also for the public. In addition, alternative postmortem diagnostic procedures have emerged. These include minimally invasive autopsies, such as endoscopic and needle tissue aspiration and autopsy, with or without ultrasonography (US) or computed-tomography (CT) guidance. Endoscopic autopsy has some drawbacks that limit its use. One of them is its need for special equipment and expertise. In addition, several anatomic regions are difficult to reach, such as the posterior mediastinum and retroperitoneum. Needle biopsies or autopsies may be an alternative for complete autopsy, especially if organs are diffusely affected. However, because this technique does not allow for inspection or dissection of the internal organs directly, valuable macroscopic information is lacking. In addition, although needle autopsy may give important information about the cause of death, not all causes for fetal or neonatal fatal outcome can be determined. When there are congenital anomalies, especially cardiovascular abnormalities, minimally invasive autopsies cannot replace conventional autopsies.
Noninvasive postmortem imaging studies, such as conventional radiography, US, CT, and MR imaging, are valuable alternatives and additions to conventional autopsy. Conventional radiography of the deceased child has been an important tool to confirm or reject the antenatal diagnosis for many decades. Especially in children with skeletal dysplasia radiography is still the primary imaging modality. With the introduction of other imaging modalities, such as US, CT, and MR imaging, also known as “virtopsies,” new indications for postmortem imaging have emerged. Of these three cross-sectional imaging modalities, US is the least favored technique, not only because it is operator-dependent and sonographers feel uneasy to examine a deceased fetus or neonate, but most of all it is never possible to have an overview of all the abnormalities present compared with three-dimensional whole-body imaging techniques, such as CT and MR imaging. CT definitely has an established role in postmortem imaging, but mainly in the somewhat older population. CT is especially helpful in children suspected of battering or in cases of death after severe trauma, including forensic cases. The low tissue contrast of CT in small fetuses or neonates limits its use, however, as an alternative for classical autopsy. Postmortem MR imaging or MR autopsy became the best alternative postmortem imaging tool because of its high spatial resolution, excellent contrast and signal-to-noise ratio, and the multitude of image contrasts that can be generated. MR autopsy consequently allows one to better delineate the different tissues of the deceased body.
This article discusses how to perform MR autopsy, including such technical determinators as field strength, and factors influencing image quality, followed by a discussion of the indications for MR autopsy. Also explored are such fetal issues as stillborns and terminations of pregnancy, and postnatal issues of natural death and unexplained death.
How to perform and interpret postmortem MR imaging
Informed consent has to be obtained from the parents, emphasizing that the examination is noninvasive and that the integrity of the body is respected. Parents should be aware of the possibility to dress their baby, as they like for the burial or cremation, avoiding metallic objects in the clothing. Necklaces and medallions, which may interact with the magnetic field, must be removed before the examination.
Parents should be informed that postmortem MR imaging cannot replace a complete autopsy; that MR imaging is only an alternative, with the major drawback that no tissue samples are obtained; and that consequently no chromosomal, biochemical, histologic, molecular, or microbiologic analyses are possible.
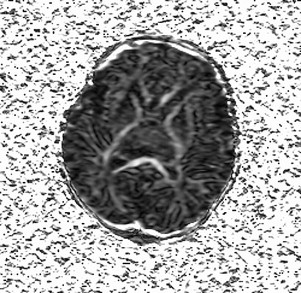
If one proposes postmortem MR imaging, it should be performed as soon as possible after the child is deceased. In the authors’ experience, the time frame depends on what the referring physician wants to know. In cases of termination of pregnancy, the physician seeks confirmation of antenatal diagnosis; in most cases this is an anatomic question or syndromal question. In most cases these questions can still be answered after a couple of days. In the case of a late third-trimester stillborn or deceased neonate, imaging should be done immediately, preferably within 24 hours, thereby avoiding refusals of consent because of cultural habits of burial or cremation within 24 hours. Another reason to scan as early as possible is to minimize the influence of maceration and autolysis of tissues within the deceased body, which decreases tissue contrast. This is especially important in cases where there is a suspicion of brain pathology. Several recent reports discuss the value of using diffusion tensor imaging (DTI) in children with severe brain malformation or those who suffered brain trauma ( Fig. 1 ). There are no evidence-based data on this subject, but if the authors use diffusion weighted imaging (DWI) or DTI for postmortem brain imaging, they try to perform the examination within 6 hours after death to limit the impact of postmortem tissue deterioration on the diffusion characteristics.
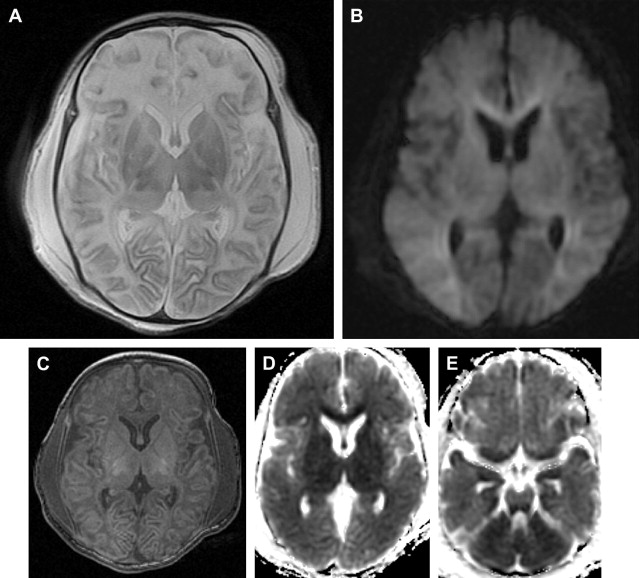
Huisman suggested a time frame of 72 hours, in which an excellent image quality is guaranteed, especially when the deceased body is immediately cooled at 4°C. In the authors’ experience, cooling has some disadvantages because it decreases the T1- and T2-weighted image contrast, which can be especially limiting in the examination of very small fetuses. Petrén-Mallmin and colleagues also showed a decreased T1- and T2-weighted image contrast related to temperature reduction. They found that T1- and T2-relaxation times of fat tissue decrease almost linearly with decreasing temperature. For other tissues, such as muscle, T1- and T2-relaxation times drop slightly to moderately. Also T2-relaxation time and signal intensity of fat and bone marrow show a significant decrease between 37°C and 10 to 20°C, the temperature frame in which the deceased body is scanned. This is important to know because most of the time imaging relies on the T2-weighted sequences because they render high spatial resolution images with optimal contrast between different tissues.
Most postmortem MR imaging is performed on 1.5-T scanners because of their wide availability. Because of the recent introduction of clinical 3-T scanners, more experience is gained with this higher field strength but no systematic studies comparing both field strengths have been published. A recently published study showed that ultra-high-field systems, up to 9.4 T, can be used for fetal postmortem imaging.
The selection of the coils depends on the size of the object. In fetuses, phased-array surface coils can be used; in neonates, the circular knee coil or phased-array head coil are available.
In most reports the imaging protocol consists of fast spin-echo T2 sequences in three orthogonal planes. The slice thickness depends on the body size and the area of highest interest. The slice thickness should not exceed 3 mm if the neonatal brain and spine are imaged. For the neonatal thorax and abdomen a slice thickness of 5 mm is sufficient. An imaging matrix of 512 × 512 is optimal, but a 256 × 256 matrix will also do in most cases. Depending on the body size the smallest field-of-view should be chosen. In most cases the entire fetus or neonate is imaged, although coverage of the extremities is not performed routinely. In some cases, after thorough consultation with the referring physician, limited studies of only the brain can be considered. In the authors’ experience the number of signal averages should be at least three to optimize the signal-to-noise ratio for diagnostic study quality. The overall scan time normally ranges between 30 and 60 minutes.
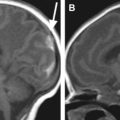
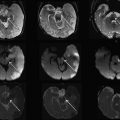
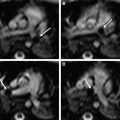
In some reports additional T2 spin density weighted sequence is suggested to increase the contrast between cerebral gray and white matter. The use of DWI/DTI for the brain can be performed in selected cases ( Fig. 2 ). The b-value should be 1000 s/mm 2 in case of a neonate but should be adjusted to the gestational age in younger babies. Like Breeze and colleagues, many experts in postmortem MR imaging believe that T1-weighted sequences add little valuable information. In the authors’ experience the three-dimensional T1 gradient echo sequence may have some advantages. First, postprocessing of the three-dimensional data set allows correcting for position differences between different parts of the body because of rigor. Second, the possibility of three-dimensional interpretation and maximum intensity projection may be helpful for reading out. Finally, T1 information of structures and focal lesions (eg, hemorrhages, meconium, and calcifications) is also obtained. This could be of help in a detailed evaluation and localization of focal pathologies in the brain, such as differentiating between germinal matrix or bleeding. In the chest T1-weighted sequences may also help to diagnose pneumonia much easier than on T2-weighted images ( Fig. 3 ). In the abdomen, liver disease is sometimes better seen on T1-weighted images ( Fig. 4 ), and meconium content in the bowel is better seen on T1- than on T2-weighted images because of the high T1-signal of meconium.