Fig. 12.1
Standard management of urinary function for all prostatectomy patients
Risk Factors
Risk factors for urinary incontinence after RP are varied and for the sake of simplicity can be broken up into demographic, disease-specific, or technique-specific/intra-operative [10, 11]. Further, they can be classified as those known preoperatively and possibly correctable, and those known immediately postoperatively. Table 12.1 summarizes these risk factors.
Table 12.1
Risk factors for post-prostatectomy urinary incontinence
Demographic factors |
Age |
BMI/physical activity |
Prostate volume |
Previous TURP |
Medical comorbidities |
Previous LUTS |
Membranous urethral length |
Disease specific factors |
Nerve-sparing surgery |
Previous RT |
Grade, stage, PSA |
Technique related factors |
Surgeon experience |
Surgical approach (Open versus MIS) |
Anastomotic technique/stricture |
Urethral fixation |
Puboprostatic sparing |
Bladder neck preservation |
Reconstruction of periurethral tissue |
Intraoperative slings |
Demographic Risk Factors
Multiple risk factors have been associated with delayed return of continence after RP including patient age, body mass index (BMI) or obesity, prostate size, membranous urethral length, presence of comorbidities, history of transurethral resection of the prostate, previous history of incontinence, or a history of lower urinary tract obstructive symptoms.
Age is one of the strongest predictors of decreased return of continence at 1 year. Licht [12], in a prospective analysis of 206 patients, showed that age greater than 65 was an independent predictor of incomplete return of urinary function after RP. Eastham et al. [8] evaluated 581 consecutive patients over a 2 year period, to determine which factors predicted an earlier return of continence. The strongest risk factor in a multivariate analysis was age. Karakiewicz [13] made a similar observation in a population based study. Age is associated with worsening continence in the general male population. Anger et al. [14] found an overall prevalence of incontinence of 17 % in men over 60 years in a population-based study. The prevalence of incontinence in the general population increased from 11 % in men 60–64 years of age to 31 % in men over the age of 85. It is no surprise, therefore, that age is consistently one of the strongest factors associated with recovery of continence after RP.
BMI or obesity has been implicated in delayed recovery of continence after RP. Mulholland et al. [15] studied the effect of BMI on urinary incontinence after RP by sending questionnaires related to voiding function to 268 consecutive patients over a 2-year period. Based on the 182 responses, no association with urinary function and body mass index was identified. However, this study had limited number of subjects and relied on correlation between degree of leakage and BMI. Anast et al. [16], on the other hand, found that BMI was indeed associated with worse urinary function, but not overall health related QoL. Their study, on a subset of 672 patients from the CapSURE registry, found that higher BMI was associated with higher rates of urinary incontinence on univariate analysis and a similar trend was observed on multivariate analysis. More recent studies have shown that obesity is indeed associated with delayed return of urinary incontinence. Wolin et al. [17] evaluated the role of physical activity and obesity in a cohort of 589 patients. They found that at 58 weeks, patients that were obese and inactive were more likely to be incontinent (59 %) than men who were non-obese and inactive (25 %) or men who were obese and active (24 %). Men who were non-obese and active had the best outcomes as related to urinary continence (16 %). Similarly, Ahlering et al. [18] noted, in a study of 100 patients undergoing robotic radical prostatectomy, that obese patients had delayed return of urinary continence.
Medical comorbidities, particularly diabetes mellitus (DM), have been associated with delayed recovery of continence after RP. Karakeiwitz et al. [19] found a strong relationship between lifetime prevalence of comorbidities and worse urinary function in a survey-based study. Teber [20] evaluated the role of DM in patients undergoing laparoscopic RP and noted that diabetic patients had later recovery of continence. In addition, a longer duration of DM was associated with later return of continence.
A history of previous TURP [8, 21–24], presence of preoperative LUTS [5, 25], and increasing prostate volume [8, 23, 26, 27] have all been suggested as risk factors, but their relation to continence recovery after RP remains controversial. Membranous urethral length has also been implicated in return of continence post RP. Membranous urethral length can be measure by performing urethral pressure profiles during urodynamics testing or via preoperative prostate imaging such as MRI. Hammerer and Huland [28] performed urodynamic evaluations in 82 men pre- and post-RP. Continent men had longer preoperative membranous urethral length than incontinent men. Similarly, Coakley et al. [29] measured membranous urethral length on preoperative endorectal-coil prostate MRI obtained in 211 consecutive patients. They noted men with shorter membranous urethral length had delayed recovery of continence than those with longer urethral length. Von Bodman et al. [30] confirmed this finding in a study which evaluated multiple soft-tissue parameters on preoperative MRI with respect to the ability to predict urinary incontinence. These authors found urethral volume and distance between the urethral edge and the levator muscles also predictive; however, the addition of these other soft tissue parameters to urethral length did not meaningfully add to the predictive accuracy of a model with urethral length alone.
Disease Specific Risk Factors
Multiple disease specific factors may be related to subsequent postoperative urinary incontinence. Nerve sparing has been implicated in early recovery of continence [5, 8, 31]. Burkhard et al. [31] evaluated 536 consecutive patients and found that attempted nerve sparing was associated with improved urinary continence. These authors found a graded level of continence; the highest in the group that underwent attempted bilateral nerve sparing and the least in the group with no nerve sparing.
Previous radiation treatment is clearly implicated in worse urinary continence after RP. Stephenson et al. [32] evaluated 100 consecutive patients that underwent RP after radiotherapy for prostate cancer and found that the rate of urinary continence was 39 % at 5-year follow-up; much lower than the rate of continence in patients that underwent RP without prior radiotherapy by the same group. Subsequently, Masterson et al. [33] showed that patients that receive pelvic radiotherapy for non-prostate malignancies have similar urinary outcomes as those that receive prostate radiotherapy. Ward et al. [34] evaluated functional outcomes after salvage RP and noted that urinary continence ranged from 43 % to 56 %, with improvement in more contemporary cases. This “learning curve” is likely related to technical factors and will be discussed in the following section. Grade, stage and preoperative PSA have generally not been implicated with recovery of urinary incontinence after RP [5, 8, 35].
Technique Related Risk Factors
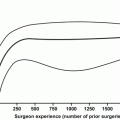
Surgeon experience and hospital volume have been associated with improved urinary outcomes. Begg et al. [36] evaluated 11,522 men that underwent RP from 1992 and 1996 and attempted to determine the effect of surgeon and hospital volume on urinary complications, including urinary incontinence. They found that hospital volume was not related to symptoms or procedures for urinary incontinence, but surgeon volume was, when controlled for case mix. Similarly, Hu et al. [37] have shown more recently that higher volume surgeons have lower rates of post RP urinary incontinence. These authors also showed that MIS surgery may be related to decreased urinary continence, although this must be considered in the setting of a new technique and may just be a manifestation of surgeons learning this technique.
Changes in surgical technique have been associated with earlier return of continence after RP. Eastham et al. [8] showed that after changing anastomotic technique, rates of urinary continence at 1 year improved from 72 % to 92 %. The technical modification used by the authors included fixing the urethral anastomosis to the periosteum of the pubic symphysis, simulating the puboprostatic ligaments. Others have shown that sparing the puboprostatic ligaments is related to improved continence. Poore et al. [38] compared 18 men who underwent puboprostatic ligament sparing RP versus 25 men who underwent standard open RP and noted that patients who underwent puboprostatic sparing had earlier return of continence (6.5 weeks vs. 12 weeks).
Bladder neck preservation is a surgical modification that has been associated with earlier return of continence by some. Multiple authors [39–41] have noted an earlier return to continence compared to other non-bladder neck sparing techniques. More recent studies, however, have shown higher rates of positive margins in groups that underwent bladder neck preservation [42] decreasing enthusiasm for this modification.
Mucosal eversion and reconstruction of the bladder neck are now considered standard parts of open RP. Furthermore, modifications in reconstruction of tissue around the urethrovesical junction have recently been proposed to improve continence. Rocco et al. [43] proposed a modification that included reconstruction of Denonvilliers’ fascia prior to performing the urethrovesical anastomosis and noted a significant improvement in time to continence in 250 patients who underwent the technique compared to 50 who did not. This group also compared this technique in 31 patients undergoing MIS RP with 31 patients undergoing MIS RP without posterior reconstruction and noted a similar hastening of continence in the group that underwent reconstruction [44]. Tewari et al. [45] compared 182 patients who underwent reconstruction of anterior and posterior periurethral tissue to 304 who underwent reconstruction of only anterior tissue to 214 who underwent no reconstruction and noted significantly shorter time to continence in the total reconstruction group.
Intrafascial dissection has been proposed as surgical modifications performed during MIS RP that lead to earlier return of continence. Neill et al. [46] compared continence rates in 240 patients who underwent laparoscopic RP with an intrafascial dissection to 270 patients who underwent standard dissection and noted earlier return of continence without any change in cancer control or potency in patients who underwent intrafascial dissection.
The use of slings at the time of RP has been attempted as a way to improve return of continence. Jorion [47] placed a strip of rectus fascia under the urethrovesical anastomosis in 30 consecutive men. He then compared return of continence to the previous 30 patients who did not undergo a sling and noted an earlier return of continence in the sling group. Jones [48] evaluated using an absorbable sling in a similar anatomic position and noted earlier return of continence, but did note a higher rate of anastomotic strictures.
Management of Post-prostatectomy Urinary Incontinence
A thorough evaluation is important when evaluating patients presenting for treatment of post-RP urinary incontinence. Figure 12.2 illustrates the management algorithm for patients with persistent post-RP incontinence. A simple history can help delineate the difference between urge urinary incontinence (UUI) and stress urinary incontinence (SUI)—an important distinction because of vastly different forms of treatment [49, 50]. UUI should be suspected in patients with significant urinary frequency, urinary urgency, or nocturia often associated with nighttime urinary incontinence. This form of incontinence often responds to medical management, whereas SUI rarely does. Regardless, both UUI and SUI are initially treated with conservative management. If conservative management fails, then additional testing in the form of urodynamics and/or cystoscopy or a trial of pharmacotherapy may be indicated.
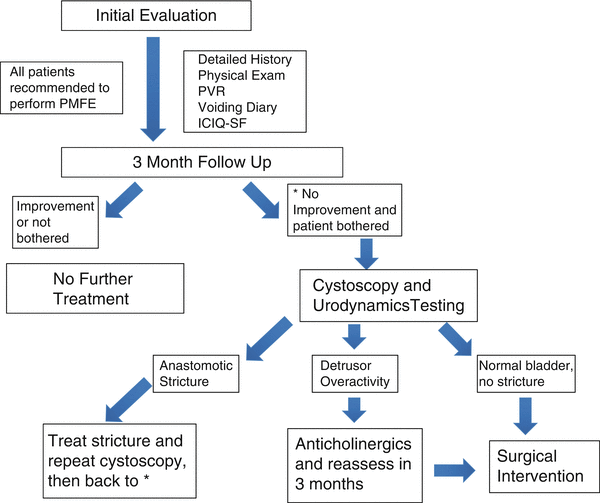

Fig. 12.2
Management of patients presenting for persistent post-prostatectomy incontinence
Conservative Management
Conservative management is important in managing urinary incontinence after RP. In general, this includes limiting fluid intake, particularly at night, avoidance of known bladder irritants, such as caffeine and alcohol, and PFME. Bladder training and timed voiding have not been shown to be useful in men [51, 52].
Pelvic Floor Muscle Exercises/Behavior Modifications
Pelvic floor muscle exercises, also called “Kegel exercises,” for the physician who popularized them, consist of intermittent voluntary contractions of the urethral sphincter muscle. If patients are unable to generate a urethral sphincter muscle contraction, aides including biofeedback might be helpful. PFME have been studied in the setting of post-prostatectomy incontinence and appear to be beneficial at hastening return of continence. A randomized control trial to evaluate the effect of PFME in men who had undergone RP used urinary continence at 3 months after surgery as the primary endpoint [53]. In this study, 88 % of the men in the treatment group achieved complete continence, measured by 24-h pad weight, significantly greater than 56 % of the men who achieved continence at 3 months in the placebo group. At 1 year, the difference between the two groups was only 14 %. Subsequently Filocamo et al. [54] randomized 300 consecutive patients who had undergone RP for clinically confined prostate cancer and showed an earlier return to continence with 74 % of the men performing PFME being dry, measured by pad usage, compared to 30 % in the untreated group at 3 months. While this difference was statistically significant, the difference at 1 year (98.7 % vs. 88 %) was not. Most men regain continence after RP; it appears that PFME can reduce time to continence, at least in the first postoperative year. Goode et al. [55] studied the effect of PFME on patients presenting with persistent post-prostatectomy urinary incontinence, defined as incontinence present after the first postoperative year. These authors randomized 208 men to one of three arm—one underwent 8 weeks of behavior therapy (PFME and bladder control strategies), the second included behavior therapy plus (addition of biofeedback and pelvic floor muscle stimulation), and a control arm (no treatment). They noted a 55 % decline in mean incontinence episodes at 8 weeks in the behavior therapy arm compared to 24 % in the control arm, a statistically significant improvement. Furthermore, they noted no significant improvement over behavior therapy with the addition of biofeedback and pelvic floor muscle stimulation. As such, a trial of pelvic floor exercises appears prudent for all men presenting for management of urinary incontinence after RP.
Biofeedback
Biofeedback has been evaluated to determine if it improves recovery of continence over PFME alone. Burgio et al. [56], studied the use of preoperative biofeedback assisted behavioral training to decrease post-RP incontinence. Participants were taught pelvic floor muscle control and received instructions in daily PFME. Patients were taught to contract the sphincter muscles during 2 to 10-s periods separated by 2–10 s of relaxation depending on initial ability. The main outcome measurements were duration of incontinence as derived from bladder diaries, severity of incontinence, distress from incontinence, and pad use. The Hopkins Symptom Checklist was used to measure psychological distress and the Medical Outcomes Study Short Form Health Survey (SF-36) was used to assess impact on QoL. They concluded that preoperative behavioral training could hasten the recovery of urine control and decrease the severity of incontinence following RP. A similar study done by Wille et al. [57], questioned the benefit of early biofeedback after RP, the outcomes measured included a 20-min pad test (an objective measure) and a urine symptom questionnaire.
Goode et al. [55] found that the addition of biofeedback to behavior therapy did not improve continence compared to behavior therapy alone, suggesting that if patients can perform PFME regularly, biofeedback has no role as adjunct therapy.
Management of UUI
Urge urinary incontinence is present in the minority of men who present for evaluation of post-RP incontinence [58, 59]. It is characterized by urinary frequency, urgency, and nocturia. Nighttime urinary incontinence is the hallmark of patients with urge urinary incontinence post-RP. The treatment of UUI in the post-RP setting has not been studied extensively, but just as in overactive bladder, anticholinergic medications have a primary role. If these medications are not efficacious or have intolerable side effects, second line treatment such as injection of botulinum toxin in the bladder or possibly sacral neuromodulation may have a role. It should be stressed, however, that data regarding these modalities and pharmacology for post-RP UUI is lacking.
Surgical Management of SUI
Surgical treatments, endoscopic or open, are usually not entertained for men with stress urinary incontinence until conservative treatments have failed. Given the natural history of urinary function recovery after RP, it is prudent to wait at least a year after surgery before undergoing evaluation and treatment for urinary incontinence. Surgical management remains the mainstay for the correction of post-RP SUI. Common surgical procedures currently used are endoscopic bulking agents, the artificial urinary sphincter, and a variety of male slings. Table 12.2 lists selected reports with the various modalities as well as the success rate.
Table 12.2
Select reports of incontinence procedures with number of patients, success rate, efficacy measures used, and whether or not complications presented
Series | # patients | Success rate | Pads/day | Questionnaires used | Complications reported |
|---|---|---|---|---|---|
Urethral bulking agents | |||||
Cummings et al. [60] | 19 | 21 % | Yes | No | |
Smith et al. [61] | 62 | 38.70 % | Yes | No | |
Westney et al. [62] | 322 | 17 % | Yes | Satisfaction score | No |
Male sling | |||||
Kaufman [64] | 42 | 63 % | Yes | No | |
Schaeffer et al. [65] | 64 | 56 % | Yes | Yes | |
Clemens et al. [66] | 66 | 53 % | Yes | Custom, satisfaction score | Yes |
Comiter [68] | 21 | 76 % | Yes | UCLA/PCI | Yes |
Ullrich and Commiter [69] | 22 | 73 % | Yes | UCLA/PCI | Yes |
Rajpurkar et al. [70] | 46 | 37 % | Yes | UCLA/PCI, satisfaction score | Yes |
Fischer et al. [71] | 62 | 58 % | No | UCLA/PCI, ICIQ-SF, IIQ-7, PGI-I, UDI-6 | Yes |
Artificial urinary sphincter | |||||
Scott et al. [74] | 34 | 79 % | Yes/no leakage | No | |
Leibovich and Barrett [80] | 417 | 88 % | Yes | Yes | |
Haab et al. [114] | 68 | 79 % | Yes | Voiding and QOL | No |
Elliot and Barrett [81] | 323 | NR | No | Yes | |
Venn et al. [82] | 100 | 84 % | Yes | Yes | |
Montague et al. [78] | 113 | 32 % | Yes | Satisfaction score | No |
Gousse et al. [79] | 71 | 58 % | Yes | Satisfaction score | Yes |
Raj et al. [83] | 554 | Variable | xx | xx | |
Lai et al. [84] | 218 | Variable | xx | xx | |
Endoscopic Management/Urethral Bulking Agents
Urethral bulking agents have been used in female SUI and have also been applied to male SUI, particularly in the setting of post-RP SUI. Glutaraldehyde cross-linked collagen has been approved by the Food and Drug Administration for the treatment of intrinsic sphincter deficiency since 1993. In males with post-RP SUI, the technique consists of endoscopic injection of collagen in the submucosa overlying or just distal to the urethral sphincter at four sites circumferentially until the urethra coapts. Collagen injection can be repeated after 4 weeks.
Cummings et al. [60] reviewed their initial series of glutaraldehyde cross-linked collagen used as an injectable bulking agent for the therapy of post-radical prostatectomy stress incontinence. Preoperative severity of incontinence was measured as mild (1–2 pads/day), moderate (3–4 pads/day) or severe (more than 4 pads/day or total incontinence). These authors reported that 58 % of patients had a “good” or “improved” result at a mean follow-up of 10.3 months. Smith et al. [61] reviewed their series of men with post-RP SUI who underwent injection of glutaraldehyde cross-linked collagen and stratified the patients as being “totally dry” or “socially continent” if they used no more than 1 pad daily. Their analysis of men who underwent collagen injection revealed that 8.1 % of the men were dry and 38.7 % of the men achieved social continence after a median of 4 injections. In a more recent review, Westney et al. [62] calculated pad usage before and after therapy. Treatment effect was stratified into quartiles of improvement of 0 % to 25 %, 26 % to 50 %, 51 % to 75 %, and 76 % to 100 %. 17 % of patients gained complete continence. Published success rates with urethral bulking agents is difficult to compare because of varying number of injections and multiple outcome measures used in studies, but ranges from 17 % to 38 % [60–62].
It should be noted that most authorities do not consider urethral bulking agents as a durable treatment for male SUI, particularly post-RP. In fact, the most recent International Consultation on Incontinence [63], a consensus meeting of incontinence experts, regarded urethral bulking agents as showing only modest success rates with low cure rates for male SUI.
Male Slings
Urethral compression procedures were introduced in 1972 when Kaufman published a series of manuscripts evaluating three different types of urethral compression techniques designed to improve continence after RP. The procedures consisted of using the penile crura with or without a buttress to compress the bulbar urethra. In his early description of this procedure he reported his success rates as excellent if there was no leakage, marked improvement in those with some leakage but no appliance and poor if a clamp was necessary. His final procedure, a silicone gel compressive implant placed just under the bulbar urethra, had a success rate, defined as dry without protection, of 63 % in 21 men with short-term follow-up [64]. These procedures, along with the success of the pubovaginal sling for female SUI, led to multiple types of male urethral slings. A male sling based on the needle suspension procedures for incontinence in females was subsequently introduced. This sling consists of three synthetic bolsters placed under the bulbar urethra and suspended above the rectus fascia in the lower abdomen via sutures through the retropubic space. Schaeffer et al. [65] reviewed their results of this bulbourethral sling for PPI. Pad usage was measured daily and the patients were stratified into dry, improved (>50 % reduction in pad usage), and wet. The success rate, defined as being completely dry, in 64 men was 56 %. Clemens et al. [66] reviewed a series of patients undergoing the same procedure and introduced a satisfaction rate (“Would you undergo the procedure all over again?”) as an outcome measure and reported a satisfaction rate of 90.2 %.
A bone-anchored variant of the male sling was reported in 2001 [67]. Initial success with this technique, defined as being dry or needing a pad for security without any episode of incontinence, was reported to be 87.5 % in 14 men followed for a mean of 12 months. Comiter [68] prospectively evaluated 21 men who underwent the bone-anchored sling surgery. Patients were stratified per their pad usage as well as their score on the UCLA/RAND Incontinence score. In this study, 76 % of men followed for a mean of 12 months demonstrated success, defined as the need for no pads.
Ullrich and Comiter [69] evaluated 22 patients with preoperative and postoperative pad usage and preoperative and postoperative urodynamic parameters, as well as UCLA/PCI questionnaire. Pad usage decrease from 4.6 to 0.74 and they found no significant change in maximum flow rate or detrusor pressure at maximum flow rate. In terms of satisfaction, they found that 73 % of patients report no or a very small problem after male sling surgery. Rajpurkar et al. [70] utilized a similar classification to assess cure in their series of 46 patients. They classified patients as “cured” if they were dry, “improved” if they were using 1–2 pads/day and “failed” if they utilized >2 pads/day. In addition to using the UCLA/PCI questionnaire, they reported patient satisfaction rates of 70 %.
More recently, Fischer et al. [71] evaluated the male perineal sling in 62 men and attempted to determine preoperative parameters that could predict success. Patients completed multiple validated questionnaires including the I-PSS (International Prostate Symptom Score), UCLA/RAND Prostate Cancer Index urinary function score, ICIQ-SF (International Consultation on Incontinence short form), IIQ-7 (Incontinence Impact Questionnaire short form), PGI-I (Patient Global Impression of Improvement), and the UDI-6 (Urogenital Distress Index short form). Success was defined by the PGI-I, patient perception of their lower urinary tract condition, as very much or much improved. Failure was defined as a little better, no change, a little worse, or much worse. As defined by the PGI-I, these authors reported a success rate of 58 %. The successes and failures as defined by the PGI-I were then compared to their pad weights and the results of the postoperative questionnaires. They found that the patients’ perception of success correlated with the 24 h pad weight as well as all the questionnaires. This study suggests that a global question is a reasonable way to measure outcomes.
A transobturator version of the male sling is now being used extensively in the USA and Europe [72]. This sling is made of polypropylene and is placed through a perineal incision and tensioned via trocars passed through a transobturator route. Initial results with this sling are comparable to initial results with the bone anchored sling. Published success rates with male slings range from 38 % to 76 % depending on outcome measures used [66–72].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree