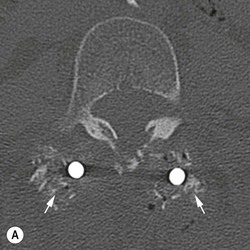
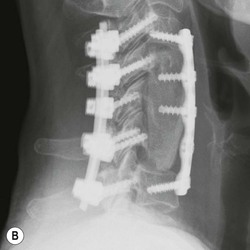
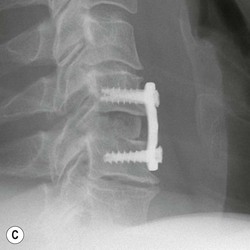
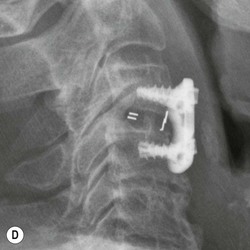
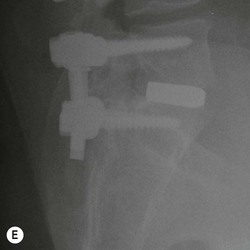
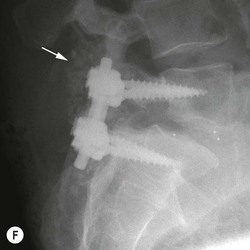
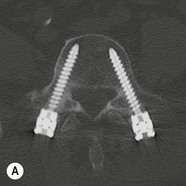
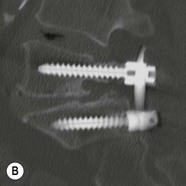
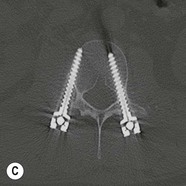
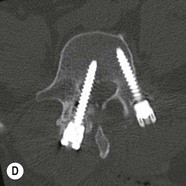
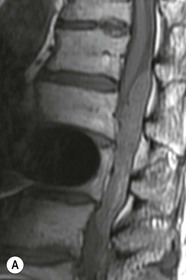
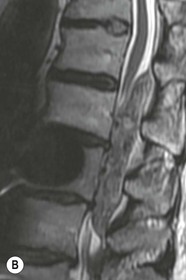
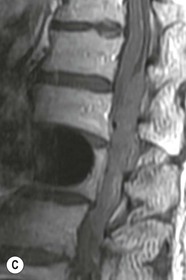
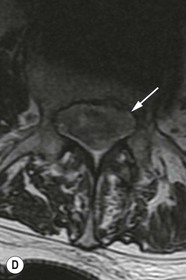
Tomasz Matys, Nasim Sheikh-Bahaei, Jonathan H. Gillard Imaging plays an important role in the assessment of the postoperative spine. The main objectives of imaging are to evaluate the alignment of the spinal column, the position of implants and the status of fusion or fracture healing, and to demonstrate potential complications in case of persistent or new postoperative symptoms.1,2 Postsurgical appearances may be complex, and knowledge of indications for surgery, type of the procedure, hardware and biomaterials used and pertinent clinical information is essential to avoid misinterpretation.3 In this chapter we discuss principles of spinal surgery and briefly review its potential complications with emphasis on these most often encountered in radiological practice. The goals of spinal surgery can be broadly categorised into three main groups: In order to achieve decompression, several surgical techniques are employed, usually in combination.1,4 Removal of intervertebral disc material is performed by discectomy or minimally invasive microdiscectomy; access to herniated disc may require removal of the margins of the lamina (laminotomy), unilateral laminar resection (hemilaminectomy) and resection of the ligamentum flavum (flavectomy). Techniques used in spinal canal decompression include laminoplasty (osteotomy of one lamina with contralateral partial osteotomy to allow formation of a unilateral gap), bilateral laminectomy with the removal of the posterior elements and deroofing of the spinal canal and/or facetectomy (excision of a part or entire facet joint). Neural foraminal decompression is achieved by foraminotomy. More extensive techniques used in the management of traumatic fractures and primary or metastatic spinal tumours include resection of one or both pedicles (pediculectomy), vertebral body (corpectomy) or entire vertebra (vertebrectomy). Stabilisation can be the primary goal of surgery, or can be performed in combination with decompressive or excision procedures that impair spine stability. In the majority of cases, a stabilisation procedure consists of instrumentation and bone grafting. Types of fixation devices used include translaminar or facet screws, and transpedicular screws in conjunction with rods, plates, hooks, wires or clamps.1 It is important to realise that metalwork, although usually left in place indefinitely, is only relied upon for temporary support until uninterrupted osseous union is achieved. Bone fusion can be promoted by a variety of graft materials.5 Autologous bone grafts are most often harvested from the iliac crest, another part of the spine, or for purely cortical bone, from the tibia or fibula. Ground-up (morselised) cancellous bone chips are used to promote osteogenesis (Fig. 58-1A), while cortical bone is used for structural support (Figs. 58-1B, C). Allograft bone substitutes are obtained from the tissue bank and include femoral rings, fibular struts and bone chips that can be used on their own or to supplement allografts. Synthetic graft substitutes include recombinant bone morphogenetic protein (BMP), demineralised bone matrix (DBX) and ceramics (tricalcium phosphate, hydroxyapatite or calcium sulphate), available in a variety of forms and consistencies. Increasingly, synthetic cages6 manufactured from metal (titanium or tantalum) or non-metallic radiolucent material such as carbon composite polymers, polyetheretherketone (PEEK) or bioabsorbable polylactic acid (PLA) are used instead of cortical bone for structural support in vertebral interbody fusion procedures (Figs. 58-1D–F). Such implants provide immediate load-bearing capacity while fusion occurs in their core packed with autologous cancellous bone, allograft or synthetic bone substitute. Spinal surgery can be performed from anterior, posterior or combined approaches. An anterior approach is primarily used in the cervical spine for procedures such as anterior cervical discectomy and fusion (ACDF), and anterior instrumentation for corpectomy, peg fracture fixation with anterior screws, cervical foraminotomy and disc replacement. The traditional open anterior approach in the lumbar spine has been more difficult, but with increasing use of minimally invasive surgery (MIS)7 and endoscopic surgical techniques this route is now more frequently used, for example for anterior lumbar interbody fusion (ALIF). A posterior approach is used in discectomy, foraminotomy, spinal canal decompression, various types of fixation using pedicle, translaminar or transfacet screws, and insertion of interspinous spacers, as well as posterior lumbar interbody fusion (PLIF) and transforaminal lumbar interbody fusion (TLIF). Anterior and posterior approaches can be combined in ‘360° fusion’ procedures in which anterior interbody fusion is accompanied by posterior stabilisation with translaminar or pedicle screws, and facet joint or intertransversal fixation. Scoliosis correction surgery may require an anterior or posterior approach, depending on individual anatomical considerations and involved spinal segment.1,2,7 Plain radiographs are commonly used in routine follow-up of the instrumented spine to assess hardware migration, loosening or breakage. Radiography is inexpensive, is quick to perform and is the only technique allowing imaging with the patient in an upright position. Anteroposterior and lateral projections can be supplemented with dynamic flexion and extension views to assess motion at the fusion site and adjacent segments suggestive of pseudoarthrosis and instability. Serial radiographs may be helpful in demonstrating subtle changes in implant positioning. More precise evaluation of implant position, alignment and integrity and fusion status is provided by CT. As the majority of implants are made of titanium, which has lower radiographic density than steel, beam hardening artefacts are generally minimal. The amount of artefact is further reduced by using helical acquisition (which also enables multiplanar reformats), high tube voltage and current, narrow collimation, thin sections and low pitch. Reconstruction with smooth or standard kernel may be preferable as bone kernel algorithm can accentuate artefacts. Average intensity projection (AIP) may be helpful in assessing hardware integrity.8 In non-implant-related complications and non-instrumented spine, CT has been largely superseded by MRI, but may still play a role in patients with claustrophobia or contraindications to entering the magnet. MRI is the technique of choice in non-hardware-related complications due to its ability to image soft tissue and neural structures, and conspicuity of contrast enhancement. It may demonstrate early complications such as haemorrhage, infection or dural leaks, and helps differentiate causes of persistent or recurrent symptoms.3,9 Protocols vary depending on institutional experience, especially with regards to routine use of fat suppression and gadolinium contrast medium—while some authors advocate a contrast-enhanced T1 sequence in all postoperative patients,3 other institutions rely on unenhanced T2-weighted images for routine assessment.10 Early postoperative appearances can be difficult to interpret and misleading due to signal changes in intervertebral disc, endplates and epidural soft tissues that may mimic the appearances of disc herniation and lead to false impression of residual disc material. Bone removal can be difficult to assess on T2-weighted images, and T1-weighted sections are better suited for this purpose.3,9 The presence of spinal implants is not a contraindication to MRI. Susceptibility artefacts from metallic implants are less problematic at lower field strengths and can be minimised by avoiding gradient-echo sequences, using fast spin-echo techniques, high receiver bandwidth, small voxel size, short TE and anterior-to-posterior frequency-encoding direction.11 Intra- and perioperative complications can be related to injury of structures and organs during approach to the surgical site, or to surgical technique including hardware misplacement. For example, an anterior approach to the cervical spine puts at risk the recurrent laryngeal nerve, with transient or permanent palsy due to excessive retraction or transection. Injury to vertebral artery is a rare but potentially catastrophic event. The most dreaded complication is the spinal cord injury; direct iatrogenic trauma is uncommon, but spinal cord function may be impaired by patient positioning or intraoperative hypotension, especially if there is a pre-existing cord compression or contusion. Paraspinal visceral structures that are at risk of injury during approach or by screws of excessive length or aberrant course include trachea, oesophagus, lung, pleura, thoracic duct, aorta and great vessels, heart, ureter, peritoneum and bowel.1,7 Imaging in cases of damage to these structures should be tailored to the clinical scenario and specific organ and injury pattern. Hardware-related complications in the perioperative period are mostly related to misplaced transpedicular screws. CT is the best technique for demonstrating screw placement, which should be wholly contained within the respective pedicles and covered by cortical bone from all sides (Figs. 58-2A, B); the screw tip should approach, but not breach, the anterior cortex of the vertebral body. Misplaced screws (Figs. 58-2C, D) may impinge on the spinal canal or neural foramen, threatening the spinal cord, cauda equina or exiting nerve roots. The reported rate of misplacement is high and may exceed 40%;1 however, clinically significant impingement or injury of neural structures is less common. The incidence of radiculopathy is reported at 7% and the nerve root is most at risk of injury or irritation if the medial or inferior border of the pedicle is violated. The need for screw removal or revision is dictated by neurological symptoms, taking into account the degree of misplacement. Early complications of spinal surgery include haemorrhage, cerebrospinal fluid (CSF) leak and infection.1,7,9 Postoperative haemorrhage is uncommon (less than 1% of patients) and usually occurs in the immediate postoperative period. In the cervical spine, expanding neck wound haematoma can lead to airway compression and is a surgical emergency;12 the role of imaging is limited as the diagnosis is usually clinical and urgent wound exploration should be performed without delay. Symptoms of spinal cord or cauda equina compression in the early postoperative period are suspicious for epidural haematoma.1 Small amounts of asymptomatic epidural haemorrhage are present in most procedures involving the spinal canal, but symptomatic haematoma requiring evacuation occurs in less than 0.5% of patients on average, being the highest in the thoracic spine at 4.5%.13 MRI is the technique of choice for identifying the extent of haemorrhage and compression of neural structures. Findings include an epidural mass with T1 signal dependent on age of the haematoma (iso- or hypointense in acute haemorrhage, hyperintense in chronic haematoma) and heterogeneously hyperintense on T2-weighted sequences, with no contrast enhancement (Fig. 58-3).
Postoperative Spine
Introduction
Principles of Spinal Surgery
Imaging Techniques in Postoperative Spine
Intraoperative and Perioperative Complications
Early Complications
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Postoperative Spine
Chapter 58