In the postpartum period, there is resolution of the unique anatomic and physiologic transformation of pregnancy. After delivery of the placenta and neonate, there is vascular redistribution of blood flow; the uterus returns to its nongravid size; and the endometrium regenerates to its prepregnancy state. The process of uterine involution continues for 6 to 8 weeks after birth, and abnormalities of uterine involution may be the cause of postpartum complications. An understanding of uterine transformation throughout pregnancy, particularly development of the placental bed, allows a better understanding of complications that can occur in the postpartum period.
During pregnancy, there is extensive fetal–maternal communication that establishes a uterine environment optimally suited to fetal development. This occurs via endocrine signals and mechanical stretch effects on the gravid uterus. Implantation of the blastocyst, which, along with progesterone, promotes endometrial decidualization. The decidua is the modified endometrium of pregnancy that contains enlarged endometrial stromal cells filled with glycogen and lipids. The trophoblasts are the cells derived from the implanting blastocyst that do not directly form the embryo but form the structures that nourish it, primarily the placenta.
The exact function of the decidua remains unclear. It has a nourishing function for the advancing fetal trophoblasts, likely accounting for the large amount of glycogen and lipid within decidualized stroma. The decidua most likely has an additional role of limiting trophoblastic invasion. The interaction between maternal decidua and fetal trophoblasts is a focus of ongoing research, with current studies suggesting that conditions such as preeclampsia, placenta accreta spectrum, and gestational trophoblastic disease are likely related to abnormal interactions between decidualized endothelium and fetal trophoblasts.
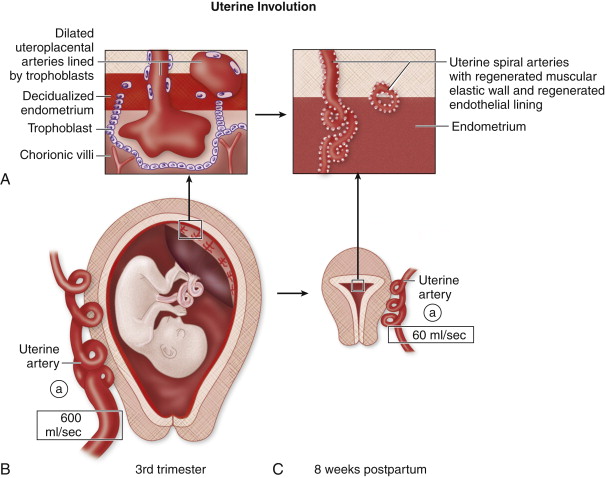
The fetus promotes maternal recognition of the pregnancy via the expression of modified trophoblastic proteins that encourage uterine quiescence so that the uterus is nonreactive to foreign tissue. In this receptive state, the uterus allows the advancement of placental trophoblasts into the decidual interstitium and lumen of the uterine spiral arteries. The uterine spiral arteries within the decidua and inner myometrium (junctional zone) are modified by the invading trophoblasts. The normal endothelial lining is replaced by trophoblasts, and the muscular layer of the elastic media is destroyed and replaced by fibrinoid material. This modification converts the contractile, high resistance uterine spiral arteries into nonmuscular, dilated, low resistance uteroplacental arteries, that ensure continuous perfusion of the developing fetus.
In the third trimester, the trophoblastic lining of the uteroplacental vessels begins to recede and becomes replaced by normal endothelium. This process continues through the early postpartum period. Similarly, there is regeneration of the elastic media that was obliterated during pregnancy. The partially replaced elastic muscular layer permits vasoconstriction during labor and delivery that, along with the clamping effect of the uterine contractions on the uterine vessels, protects the mother from hemorrhagic complications. At the time of delivery, uterine contractions decrease uterine arterial blood flow significantly while maintaining relatively steady umbilical artery flow. After delivery, postpartum involution of the uterus involves several processes similar to wound healing with an associated inflammatory component. The relative ischemia of the myometrium during contractions contributes to the inflammatory component of the postpartum uterus that includes a prominent leukocytic infiltrate within the endometrium and inner myometrium. The inflammation around the uteroplacental vessels initiates an endarteritis that promotes thrombosis and helps control postpartum bleeding from dilated residual uteroplacental arteries.
The placenta separates from the uterine surface at the basal layer of the decidua. A variable portion of the basal layer remains attached to the underlying myometrium. After delivery the basal layer is infiltrated with blood and leukocytes and becomes covered with a reticular layer of fibrin and clot that helps control bleeding.
The enlarged uteroplacental vessels of pregnancy are severed when the placenta separates from the uterus. Myometrial contractions compress the vessels, causing thrombosis to stop ongoing hemorrhage. A hypotonic uterus that cannot generate enough force to clamp the vessels can lead to fatal hemorrhage even in the presence of normal maternal coagulation mechanisms.
Throughout the postpartum period, the dilated uteroplacental vessels are transformed into smaller caliber uterine spiral arteries. Blood loss during parturition contributes to a significant decrease in uterine artery flow after pregnancy. The hypertrophied myometrium resumes its pregravid state, and the uterus decreases in size ( Figure 25-1 ).
Alterations in the complex process of uterine involution can lead to postpartum complications, including postpartum hemorrhage (PPH), infection, thromboembolic events, and complications related to cesarean delivery. Postpartum complications occur in approximately 10% of combined spontaneous vaginal and cesarean births, and are a major cause of maternal morbidity and mortality worldwide.
Normal Involution
The puerperium is loosely defined as the period of approximately 6 to 8 weeks after childbirth when the uterus returns to its pregravid state. The normal postpartum appearance of the uterus has been studied by ultrasound (US), computed tomography (CT), and magnetic resonance imaging (MRI). Uterine involution is a dynamic process with serial changes over the course of 8 weeks.
Uterine size deceases dramatically from a volume of approximately 1000 g after delivery to 500 g by the end of the first week, and to 100 g by 8 weeks. The position of the uterus adjusts to the change in size. Immediately after delivery the uterine fundus is above the brim of the pelvis and is heavy. There is slight retroflexion of the immediate postpartum uterus as a result of its size, heavy fundus, and relatively thinned isthmus. As the uterine fundus returns to the pelvis, it tends to resume an anteverted position. The increased size of the postpartum uterus coupled with its edematous state allows for fairly good sound transmission and adequate evaluation with transabdominal US in the first 2 weeks. After approximately 2 weeks, transvaginal US is usually better suited to the size and position of the uterus.
The myometrium may have variable echogenicity. The inner myometrium may be hyperechoic in the early postpartum period, making it potentially difficult to differentiate from the echogenic endometrium ( Figure 25-2 ). There may be focal areas of enhanced vascularity, most commonly a vascular pedicle extending along the full thickness of the myometrium to the endometrial cavity. This may be seen up to 6 weeks postpartum, and is thought to occur at the placental site.
Evaluation of the Doppler characteristics of the uterine arteries in the postpartum period has found variable results. There is agreement that there is some degree of increased flow resistance immediately after delivery compared with the low resistance of the uteroplacental arteries during pregnancy. Some investigators, however, report a return to baseline high resistance flow and waveform in most women by 4 postpartum weeks, whereas others suggest that resistive indices remain lower in postpartum patients beyond 8 weeks compared with nonpregnant patients.
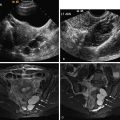
In the immediate postpartum period there is some degree of complex fluid within the endometrial canal. Most reports suggest that 24 to 48 hours after delivery there is a small to moderate amount of heterogeneous material distending the cervical canal and lower uterine segment, whereas the upper endometrial cavity is usually empty and seen as a thin, slightly irregular echogenic structure. The irregular contour of the endometrium immediately after delivery is likely the result of the irregular surface of the basal decidua after placental separation and also caused by the irregular meshlike deposition of fibrin and clot that forms along the basal surface after the placenta is delivered.
Fluid begins to accumulate in the upper endometrial cavity between 1 and 2 weeks after delivery. The normal healing process involves necrosis and shedding of the decidual lining after adequate hemostasis has been achieved immediately after delivery of the placenta. Therefore in the second postpartum week, echogenic material that represents the necrotic decidual cast can appear as an avascular echogenic mass distending the uterine cavity.
Echogenic foci can be a normal finding in the postpartum endometrial cavity and are likely related to gas or echogenic clot ( Figure 25-3 ). Echogenic foci suggestive of gas are seen in 5% to 21% of asymptomatic postpartum women, most commonly in the immediate postpartum period but reported as late as 3 weeks postpartum. Air may enter the endometrial cavity through an open patulous cervix after delivery and can therefore be seen with some frequency in normal patients.

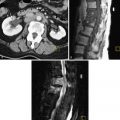
CT of the normal postpartum pelvis demonstrates an enlarged uterus with thickened myometrium ( Figure 25-4 ). Within the uterus, there may be a small amount of intracavitary fluid of variable density, and there may be foci of gas. There should not be any evidence of contrast extravasation into the uterine cavity. The uterine and ovarian vessels are larger than normal and more easily seen because of increased blood volume that occurred throughout pregnancy and has not yet returned to normal. The uterine vessels are seen as tubular-enhancing structures in the parametrium. The ovarian veins extend superiorly from the suspensory ligaments of the ovaries. The ovarian veins are lateral to the ureters within the pelvis, but they cross over the ureters at the level of the inferior mesenteric artery origin and lie medial to the ureters in the upper abdomen. Gas has been reported in the symphysis pubis and sacroiliac joints in up to 42% of patients immediately postpartum.

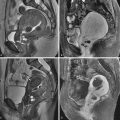
Studies evaluating the appearance of the postpartum uterus on MRI have shown high T1 and T2 signal within the endometrial canal in the first week after delivery as a result of subacute clot. Serial changes in MRI appearance include distention of the lower uterine segment with mixed signal collection at 30 hours postpartum with progression to an empty endometrial cavity in all patients at 1 week postpartum. Direct comparison with findings on US is limited because different time points have been used in various studies to evaluate serial changes. Other serial changes seen by MRI include prominent myometrial vessels and increased T2 signal within the cervical stroma, both present at 30 hours postpartum and both resolving throughout the puerperium. A study of MRI in the postpartum period showed that the junctional zone, normally seen as a thin low signal band on T2-weighted images around the endometrium in nonpregnant patients, was not seen in any patients before 2 weeks postpartum ( Figure 25-5 ). The junctional zone becomes visible later in the postpartum period and was seen in all patients by 6 months after delivery; however, even at 6 months when it generally is seen again, it may be slightly thickened and ill defined. This observation suggests judicious use of junctional zone criteria for the diagnosis of adenomyosis in the extended postpartum stage for an uncertain duration of time. Given the trophoblastic invasion of the junctional zone during pregnancy, this observation also raises questions about the physiologic involution of the junctional zone late in the postpartum period.

Postpartum Hemorrhage
PPH is defined as an estimated blood loss greater than 500 mL after vaginal delivery and greater than 1000 mL after cesarean delivery. Blood loss is difficult to estimate visually, and alternatively PPH can be defined as a 10% decrease in hematocrit from admission or blood loss requiring transfusion. PPH is categorized as primary or secondary depending on the onset of bleeding. Primary or early PPH is defined as hemorrhage occurring within 24 hours of delivery, whereas secondary or late PPH occurs between 24 hours and 6 to 8 weeks postpartum.
Primary Postpartum Hemorrhage
Primary PPH occurs in 5% of all pregnancies and is the leading cause of maternal mortality worldwide, accounting for up to 60% of maternal deaths in developing countries. Primary PPH is caused by uterine atony in at least 80% of cases. Other causes of primary PPH include lacerations of the cervix or perineum, retained placental fragments associated with placenta accreta, and coagulopathy, which may be a primary condition or a secondary response to extensive hemorrhage.
Any condition that interferes with optimal uterine contraction is associated with increased risk of uterine atony and includes prolonged labor, high parity, retained placenta, and uterine overdistention secondary to multiple gestations, polyhydramnios, and macrosomia. Uterine blood flow after delivery is approximately 600 mL/min; therefore uterine atony may be a life-threatening condition. Routine imaging is not performed when the clinical diagnosis of uterine atony is clear, which includes a boggy, noncontracted uterus associated with heavy bleeding.
CT may, however, be helpful if standard treatment is unsuccessful or if the clinical scenario is unclear. CT may confirm the diagnosis of uterine atony by demonstrating contrast pooling within the endometrial cavity, or CT may reveal an alternative diagnosis. CT may show a large pelvic hematoma or an extrauterine site of contrast extravasation that would help guide angiographic evaluation and embolization. The possibility of superimposed disseminated intravascular coagulation (DIC) may be suggested by the presence of a hematocrit sign. The hematocrit sign is described as a fluid–fluid level within a hematoma and has been reported as more commonly seen in patients with primary PPH and DIC.
Treatment of uterine atony includes administration of uterotonic agents, such as oxytocin and prostaglandin E2 analogs, blood transfusion, uterine artery embolization or ligation, and, if necessary, hysterectomy.
Lacerations, related to the trauma of delivery, may occur from the cervix to the perineum. This is suspected when there is primary PPH despite a firm, contracted uterus. Visual and manual evaluation will usually reveal the source, and imaging is rarely used. If the source of bleeding is not clinically evident, CT is recommended to evaluate for a concealed hematoma, although US may also identify pelvic hematomas ( Figure 25-6 ).

Secondary Postpartum Hemorrhage
Secondary PPH occurs in approximately 1% of pregnancies. Secondary PPH is less well documented than primary PPH because conservative treatment is usually successful, and there is less mortality associated with secondary PPH than primary PPH. Although there may be overlap in the causes of primary and secondary PPH, secondary PPH is most commonly caused by retained products of conception (RPOC), subinvolution of the placental bed, and endometritis (usually associated with RPOC if there is associated bleeding).
Subinvolution
RPOC is discussed in Chapter 23 . There is increasing recognition of subinvolution of the placental bed as a clinically important cause of secondary PPH. Normal involution is the transformation of the dilated, modified uteroplacental arteries of pregnancy to the contracted uterine spiral arteries of the nongravid uterus. Subinvolution is defined as failure of obliteration of uteroplacental arteries after pregnancy in the absence of retained chorionic villi.
The prevalence of this condition is not clear because subinvolution has not been universally recognized and has been difficult to confirm without a hysterectomy specimen. One study showed that RPOC accounted for 27% of cases of delayed PPH, and subinvolution was the cause of 18% of cases. Several studies looking at abnormal vaginal bleeding in women of reproductive age found an incidence of subinvolution in up to 5% of patients weeks to months after a pregnancy. Subinvolution is likely underreported because the diagnosis has traditionally been made on histology. It may be a difficult diagnosis to make with curettings, and fortunately, hysterectomy specimens are rare in this clinical setting.
The etiology and pathophysiology of subinvolution are not clearly understood. Subinvolution is caused by failed obliteration of the uteroplacental arteries, and it occurs in the absence of retained fetal tissue and chorionic villi. The persistent dilated uteroplacental arteries have not undergone hyalinization, thrombosis, and recanalization into normal caliber uterine spiral arteries. As a result, there is continuous high volume, low resistance flow within the dilated arteries just below the endometrial surface, which can rupture and cause postpartum bleeding.
It has been suggested that subinvolution is caused by an abnormal interaction between fetal trophoblasts and maternal tissue on an immunologic basis. Subinvolution and preeclampsia may represent opposite ends of the spectrum where the underlying mechanism is an altered immunologic response of the maternal tissue to the invading trophoblasts.
In preeclampsia, altered maternal–fetal interaction early in pregnancy leads to inadequate remodeling of the uterine spiral arteries into uteroplacental vessels with ensuing hypertension, whereas in subinvolution, faulty regression of the uteroplacental vessels late in pregnancy and after delivery may account for delayed PPH.
US shows dilated tubular anechoic or hypoechoic structures in the inner myometrium without evidence of echogenic material in the endometrial cavity or an associated echogenic mass in the inner myometrium ( Figure 25-7 ). Color Doppler shows marked mosaic flow within the tubular structures. Spectral Doppler evaluation shows low resistance flow characteristic of trophoblastic tissue with variable reported velocities. On angiography, subinvolution may present as hypertrophied uterine arteries with rapid opacification of uterine parenchyma. Drainage is into normal pelvic veins, and there is no evidence of early venous filling to suggest an abnormal arteriovenous communication.

The imaging differential diagnosis for subinvolution is primarily uterine arteriovenous malformation (AVM), although gestational trophoblastic disease (GTD) and RPOC are also considerations. There is significant overlap in the sonographic appearance of subinvolution and uterine AVM. Future studies will likely define imaging criteria that will help in the imaging diagnosis of subinvolution and differentiation from uterine AVM.
From a clinical standpoint, subinvolution and uterine AVM are managed in the same way, and treatment is based on the severity of bleeding. Mild hemorrhage may be treated conservatively, whereas more severe hemorrhage requires uterine artery embolization or possibly hysterectomy to control bleeding. It is important to differentiate a vascular abnormality from RPOC because RPOC is often treated with curettage, and uterine instrumentation may aggravate bleeding that is caused by an underlying vascular abnormality. US is important to exclude the possibility of a vascular lesion as the cause of PPH because there are reports of massive hemorrhage from dilatation and curettage (D&C) of a vascular lesion, including subinvolution. Therefore the presence of a vascular lesion is considered a relative contraindication to D&C.
Arteriovenous Malformations
Uterine AVMs may be congenital or acquired. An AVM is defined as an abnormal communication between arteries and veins with the absence of an intervening capillary bed. Congenital AVMs are rare; however, acquired AVMs are reported with increasing frequency.
There is growing evidence that acquired AVMs are seen in women with recent pregnancies. In one study, all 30 patients diagnosed with a vascular malformation by US had a history of miscarriage, D&C, or term pregnancy within 6 months of the sonographic diagnosis. Acquired AVMs may be the result of vessel injury caused by surgery or instrumentation. Given the high association of AVM with pregnancy, however, there is speculation that many reported AVMs are in fact subinvolution of the placental bed and may share a similar etiology related to delayed transition of uteroplacental vessels to normal uterine spiral arteries. Recent studies have shown that only 10% to 21% of cases of AVMs diagnosed by US are confirmed as true AVMs by angiography. Furthermore, up to 50% of AVMs seen by US have been found to disappear on follow-up evaluation weeks to months later, a finding that is more suggestive of subinvolution ( Figure 25-8 ).

Patients with an AVM may present with intermittent, severe vaginal bleeding with a history of previous uterine instrumentation, surgery, or trauma. Alternatively, sonography may reveal a lesion that appears to be an AVM in a patient who is asymptomatic.
AVMs may be seen on US as cystic areas in the myometrium or as subtle areas of myometrial heterogeneity that show marked vascularity with color Doppler and low resistance, high velocity flow by spectral Doppler ( Figure 25-9 ). The gray scale appearance of AVM may be identical to that of subinvolution with dilated tubular hypoechoic structures in the inner myometrium. Spectral Doppler shows variable reported peak systolic velocities (PSV) ranging from 25 to 200 cm/s; however, it remains unclear as to how many of these reported AVMs seen by US are in fact subinvolution. At this time, there are no clear sonographic criteria that differentiate a true uterine AVM from subinvolution. If the patient is stable, follow-up US evaluation should be considered and can help confirm subinvolution by demonstrating spontaneous resolution of the sonographic findings. Both of these vascular lesions are associated with low resistance flow resulting from arteriovenous shunting in AVMs and persistent trophoblastic flow in subinvolution, and there is a wide range of reported PSVs for both conditions. The differential diagnosis of AVM on US includes subinvolution, RPOC, and GTD.