Although conventional contrast-enhanced MR imaging remains the standard-of-care imaging method in the posttreatment evaluation of gliomas, recent developments in therapeutic options such as chemoradiation and antiangiogenic agents have caused the neuro-oncology community to rethink traditional imaging criteria. This article highlights the latest recommendations. These recommendations should be viewed as works in progress. As more is learned about the pathophysiology of glioma treatment response, quantitative imaging biomarkers will be validated within this context. There will likely be further refinements to glioma response criteria, although the lack of technical standardization in image acquisition, postprocessing, and interpretation also need to be addressed.
Key points
- •
Use of the MacDonald criteria has been the most widely used method to assess therapeutic response in high-grade gliomas and involves examining changes in contrast-enhancing area, typically on conventional contrast-enhanced magnetic resonance (MR) imaging.
- •
Several limitations of the MacDonald criteria have been identified since their introduction in 1990. The most critical limitation rests on its reliance on contrast enhancement as a criterion of therapeutic response. Although contrast enhancement is a sensitive marker of blood-brain barrier disruption, it is not a specific finding of active tumor, and can be the result of many other processes including treatment-related effect, ischemia, seizure, inflammation, and postoperative changes.
- •
With the recent recognition of the entities of pseudoprogression and pseudoresponse associated with chemoradiation with temozolomide and antiangiogenic agents, respectively, the neuro-oncology community has been forced to reevaluate traditional imaging criteria of treatment response.
- •
The latest Response Assessment in Neuro-Oncology Working Group recommendations to address limitations in the MacDonald criteria are reviewed. In addition, the most recent advances in quantitative biomarker development using advanced imaging modalities are highlighted. However, until these techniques are thoroughly validated, conventional contrast-enhanced MR imaging follow-up should remain the standard of care.
Introduction
Gliomas represent the most common adult primary brain malignancy with an annual incidence of about 4 to 5 per 100,000. The prognosis for glioblastoma in particular remains dismal. Postoperative radiation therapy has been an integral part of the treatment of high-grade gliomas (HGGs) since the 1970s. Over the next 2 decades, innovations in computed tomography (CT) and magnetic resonance (MR) imaging improved both brain tumor characterization and radiotherapy techniques. However, further attempts using alternative methods of radiotherapy failed to improve outcomes. In 2005, Stupp and colleagues showed improved survival with the addition of concurrent and adjuvant temozolomide (TMZ) to radiotherapy and this regimen, combined with maximal surgical resection, has since become the current standard of care for newly diagnosed glioblastoma. In 2009, the antiangiogenic agent bevacizumab received US Food and Drug Administration approval for the treatment of recurrent/progressive glioblastoma. Therapeutic assessment of high-grade gliomas (HGGs) relies on patient survival or, in cases of recurrent tumor, often the radiographic response rate or progression-free survival (PFS). The adoption of chemoradiation with TMZ and antiangiogenic agents into the therapeutic armamentarium has resulted in a reevaluation of conventional contrast-enhanced MR imaging and response criteria.
The most widely used method to assess therapeutic response in HGGs has been to examine changes in contrast-enhancing area, typically on conventional contrast-enhanced MR imaging. Progression on imaging is defined as either a 25% increase in the size of enhancement or new foci of enhancement ( Table 1 ). In addition, corticosteroids and neurologic status are also taken into consideration. Taken together, this schema is known as the MacDonald criteria. When proposed in 1990, these criteria represented a shift from a subjective evaluation of clinical and radiologic data toward a more objective, image-based methodology.
| Response | Criteria |
|---|---|
| Complete response |
|
| Partial response |
|
| Stable disease |
|
| Progression |
|
Introduction
Gliomas represent the most common adult primary brain malignancy with an annual incidence of about 4 to 5 per 100,000. The prognosis for glioblastoma in particular remains dismal. Postoperative radiation therapy has been an integral part of the treatment of high-grade gliomas (HGGs) since the 1970s. Over the next 2 decades, innovations in computed tomography (CT) and magnetic resonance (MR) imaging improved both brain tumor characterization and radiotherapy techniques. However, further attempts using alternative methods of radiotherapy failed to improve outcomes. In 2005, Stupp and colleagues showed improved survival with the addition of concurrent and adjuvant temozolomide (TMZ) to radiotherapy and this regimen, combined with maximal surgical resection, has since become the current standard of care for newly diagnosed glioblastoma. In 2009, the antiangiogenic agent bevacizumab received US Food and Drug Administration approval for the treatment of recurrent/progressive glioblastoma. Therapeutic assessment of high-grade gliomas (HGGs) relies on patient survival or, in cases of recurrent tumor, often the radiographic response rate or progression-free survival (PFS). The adoption of chemoradiation with TMZ and antiangiogenic agents into the therapeutic armamentarium has resulted in a reevaluation of conventional contrast-enhanced MR imaging and response criteria.
The most widely used method to assess therapeutic response in HGGs has been to examine changes in contrast-enhancing area, typically on conventional contrast-enhanced MR imaging. Progression on imaging is defined as either a 25% increase in the size of enhancement or new foci of enhancement ( Table 1 ). In addition, corticosteroids and neurologic status are also taken into consideration. Taken together, this schema is known as the MacDonald criteria. When proposed in 1990, these criteria represented a shift from a subjective evaluation of clinical and radiologic data toward a more objective, image-based methodology.
| Response | Criteria |
|---|---|
| Complete response |
|
| Partial response |
|
| Stable disease |
|
| Progression |
|
Limitations of the MacDonald criteria
Since their introduction, several limitations of the MacDonald criteria have been identified. These limitations include interobserver variability, failure to measure nonenhancing portions of tumor (particularly significant for evaluation of low-grade gliomas [LGGs]), difficulty in measuring tumors with irregular shapes, lack of guidance in the evaluation of multifocal tumors, assessment of progression after gross total resection of all enhancing tumor, and difficulties with measuring enhancing lesions in the walls of cysts/surgical cavities because the cysts/cavities may be incorporated into the tumor size measurement.
The most critical limitation rests on the MacDonald criteria’s reliance on contrast enhancement as a criterion of therapeutic response. Although contrast enhancement is a sensitive marker of blood-brain barrier (BBB) disruption, it is not a specific finding of active tumor, and can be the result of many other processes including treatment-related effect, ischemia, seizure, inflammation, and postoperative changes. In addition, corticosteroid dosage can complicate assessment in that this can physiologically decrease the amount of contrast enhancement. In particular, the recent recognition of the entities of pseudoprogression (PsP) and pseudoresponse (PsR) associated with chemoradiation with temozolomide and antiangiogenic agents, respectively, has emphasized the need to reevaluate traditional imaging criteria of treatment response.
Therapeutic evaluation after chemoradiation with TMZ in HGG- radiation necrosis and PsP
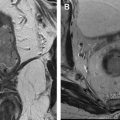
Radiation necrosis (RN), and the recently recognized PsP, are forms of treatment-related enhancement mimicking tumor progression that can occur following chemoradiation with TMZ. They pose significant problems for treating physicians because they are essentially indistinguishable from tumor progression using conventional MR imaging methods ( Fig. 1 ). Further complicating matters is the possibility to have both coexisting tumor and therapy-induced necrosis in the same enhancing lesion.
RN
Radiation-induced injury represents not a single instantaneous event but a dynamic, complex process that develops over time, with a significant amount of tissue damage occurring hours to days after the initial injury. These processes can include vascular injury with vasogenic edema, glial and white matter damage, effects on the fibrinolytic enzyme system, and immune mechanisms. Radiation effects are usually divided into acute, subacute, and late effects. Vasodilatation, damage to the BBB, and edema are thought to underlie both acute and subacute types of radiation injury.
Late radiation effects include RN as well as other processes such as leukoencephalopathy, vascular lesions such as moyamoya syndrome and lacunar infarcts, parenchymal calcifications, and enhancing white matter lesions. RN typically occurs months to years following therapy and may be progressive and irreversible. The incidence of RN is unclear, but may be as high as 24%. Both the volume of brain irradiated as well as the radiation dose delivered are important factors, particularly when doses are higher than 65 Gy in fractions of 1.8 to 2.0 Gy. In RN, blood vessels show fibrinoid necrosis with surrounding perivascular parenchymal coagulative necrosis. Endothelial injury from radiation results in fibrinoid necrosis of small vessels, endothelial thickening, hyalinization, and vascular thrombosis. In contrast, recurrent tumor shows vascular proliferation and angiogenesis without vascular luminal obliteration. Patterns described as “soap bubble” or “Swiss cheese” have been described on conventional contrast-enhanced MR imaging in RN; however, these appearances cannot reliably differentiate between tumor recurrence and RN. Advanced MR imaging techniques (discussed later) have been examined to better characterize RN. However, no technique is widely accepted and this remains an active area of research.
The treatment of RN ranges from observation to medical and surgical therapy. Medical treatment incorporates corticosteroids because of their ability to counteract BBB disruption from radiation-induced vascular endothelial injury. Because there seems to be increased vascular endothelial growth factor (VEGF) and microvascular permeability in RN, there is some evidence to suggest that RN may be responsive to bevacizumab treatment. Hyperbaric oxygen treatment has also been explored as a treatment option because it may stimulate angiogenesis and restore the vascular supply injured by radiation. Medically intractable cases may require surgical resection if the lesion is surgically accessible and will not result in significantly increased morbidity.
PsP
While PsP may occur following radiotherapy alone, there has been a greater focus on PsP since the recent adoption of chemoradiation with TMZ as the standard of care for glioblastoma. Although PsP currently lacks a strict definition, in general it refers to an increase in contrast enhancement within the first 3 to 6 months after chemoradiation that is the result of treatment-induced changes rather than true early progression (TEP). The time period of its occurrence is earlier than after radiotherapy alone and, as such, it may represent a mild, self-limiting variant of RN. However, there is evidence to suggest that PsP and RN are distinct entities with PsP representing a combination of treatment effect on residual tumor cells and disruption of the BBB, whereas RN represents radiation damage to the peritumoral white matter. As such, some have argued that the term PsP should replace the outdated term early radionecrosis.
Without a change in therapy, PsP may improve or disappear, although in some cases it may remain persistent. Although most patients with PsP are asymptomatic, as many as one-third may need surgery, increased steroids, or possibly antiangiogenic therapy because of symptoms from mass effect.
PsP seems to be a common occurrence, with incidence estimates of approximately 20% to 30% and seems to be more frequent in patients with a methylated O 6 -methylguanine–DNA methyltransferase (MGMT) promoter. More recent analyses seem to support this incidence estimate. However, because of varying definitions, uncertainties in image interpretation, as well as variations in the quality and design of studies, the true incidence of PsP is still not clear. Clarke and Chang estimate that about half of all patients with glioblastoma develop worrisome contrast-enhancing lesions on follow-up MR imaging after chemoradiation and that many are the result of PsP rather than TEP. In 2011, a retrospective study of the first postradiotherapy scans in 321 patients with glioblastoma treated with chemoradiation with TMZ was reported from a major brain tumor referral center. The investigators reported that this was, to date, the largest cohort of patients examined to determine whether conventional MR imaging could distinguish PsP from TEP. Suspicious new or enlarging enhancing lesions were found in 93 patients. Of these, 30 patients (32.3%) were determined to have PsP (confirmed pathologically in 6 cases). This incidence is consistent with several prior reports. Eleven conventional MR imaging signs were evaluated and only subependymal enhancement could predict TEP with 38.1% sensitivity, 93.3% specificity, and 41.8% negative predictive value. The remaining 10 signs were not predictive and there was not a sign with a sufficiently high negative predictive value for PsP. As such, the investigators concluded that conventional MR imaging signs have limited usefulness to diagnose PsP and that an alternative imaging biomarker is needed.
PsP and MGMT promoter methylation status
The MGMT enzyme is a DNA repair protein that provides resistance to the alkylating drug TMZ by removing alkyl groups (such as those placed by chemotherapeutic alkylating agents) from the O 6 position of guanine. Epigenetic silencing of the MGMT gene by promoter methylation causes a loss of its expression, thus inhibiting the DNA repair mechanism and resulting in chemotherapy-induced cytotoxicity and apoptosis. The status of the MGMT promoter represents a potentially useful marker to complement imaging because PsP is more frequently seen in cases with a methylated promoter than in those with an unmethylated promoter. Numerous studies have also shown that methylated MGMT promoter status is associated with improved survival with TMZ treatment.
Clinical implications of PsP
The inability of conventional MR imaging methods to differentiate PsP from TEP limits the validity of PFS as a primary end point (unless pathologic confirmation of TEP is made) and confounds the design of salvage clinical trials. If a patient does not respond to initial therapy with TMZ then treatment should be changed quickly, often into a clinical trial. However, if a patient has developed PsP, then altering management prematurely terminates an effective therapy, and, because PsP tends to improve on its own, can result in a falsely high response rate and PFS as well as lending a false attribution of efficacy to the new agent.
Antiangiogenic therapy and PsR
Cancers grow beyond their initial local blood supply by developing deregulated angiogenesis, which allows tumors to acquire an abnormal vasculature composed of dilated, tortuous, and hyperpermeable vessels. This results in irregular, inefficient perfusion of tumors, and ultimately hypoxia and necrosis. At first, the mechanism of antiangiogenic agents against tumors was thought to be via reduction or elimination of tumor vasculature, effectively starving the tumor. However, clinical studies have shown an absence of a clear dose-response relationship as well as a lack of benefit without concomitant cytotoxic therapy. In addition, the resulting hypoxia caused by elimination of blood vessels should result in decreased effectiveness of chemotherapy. However, this has also not been seen in clinical studies. As a result, an alternative mechanism termed vascular normalization has been proposed. In vascular normalization, antiangiogenic agents cause a decrease in vessel diameter and permeability as well as thinning of the abnormally thick basement membrane.
Treatment with antiangiogenic agents directed against VEGF, such as bevacizumab, can cause a rapid decrease in enhancement caused, at least in part, by a decrease in microvascular permeability secondary to vascular normalization, rather than a true antitumoral effect. This process has become known as PsR. A decrease in contrast enhancement has been documented as early as 24 hours following a single dose of the pan-VEGF tyrosine kinase inhibitor cediranib, and discontinuing the drug leads to a rapid reversal in enhancement, which lends credence to this notion.
Some patients treated with angiangiogenic agents may also experience nonenhancing tumor progression manifesting as increasing T2/fluid-attenuated inversion recovery (FLAIR) signal abnormality ( Fig. 2 ). Antiangiogenesis therapy may stimulate tumor progression through vascular cooption and develop an invasive, nonenhancing phenotype. This process could help explain the large disparity between high response rates in recurrent glioblastoma and modest, if any, survival benefit.
Regardless of whether there is a PsR or true antitumoral response, vascular normalization with an associated decrease in vasogenic edema may result in decreased morbidity and steroid usage.
Clinical Implications of PsR
There are currently no validated predictive biomarkers for any antiangiogenic agent in cancer. Radiologic responses in patients being treated with antiangiogenic agents must be viewed with skepticism. As with PsP, the possibility of PsR limits the usefulness of PFS as a primary end point in clinical trials. Furthermore, changes in T2/FLAIR signal abnormality have not been explicitly addressed by the traditional MacDonald criteria and should be addressed in updated criteria.
RANO criteria
The Response Assessment in Neuro-Oncology (RANO) Working Group is a multidisciplinary, international collaborative effort whose goal is to provide expert consensus opinion regarding the development of new standardized response criteria for brain tumor clinical trials. In 2010, given concerns raised by PsP and PsR, the RANO Working Group attempted to address some of the limitations of the MacDonald criteria. Despite many advances in functional MR imaging techniques (discussed later), there is currently insufficient evidence to incorporate them into routine response criteria for use in clinical trials/practice and, therefore, conventional follow-up contrast-enhanced MR imaging remains the standard-of-practice imaging technique. As a result, the determination of PsP by MR imaging alone is inherently a retrospective process. Furthermore, other end points such as quality-of-life measures and neuropsychological testing may be incorporated into response criteria as these metrics are developed and validated.
A summary of the proposed RANO response criteria is given in Tables 2–4 .
| First Progression | Criteria |
|---|---|
| Progressive disease <12 wk after completion of chemoradiation | Progression can only be defined using imaging if: There is new enhancement outside the radiation field (beyond the high-dose region or 80% isodose line) or if there is unequivocal evidence of viable tumor on histopathologic sampling (eg, solid tumor areas, ie, greater than 70% tumor cell nuclei in areas; high or progressive increase in MIB-1 proliferation index compared with prior biopsy; or evidence for histologic progression or increased anaplasia in tumor) Because of the difficulty of differentiating TEP from PsP, clinical deterioration alone, in the absence of histologic or radiographic confirmation of progression, is not sufficient for definition of progressive disease in the first 12 wk after completion of concurrent chemoradiation |
| Progressive disease ≥12 wk after completion of chemoradiation |
|
| Response | Criteria |
|---|---|
| Complete response |
|
| Partial response |
|
| Stable disease |
|
| Progression |
|
a Stable corticosteroid doses include patients not taking corticosteroids.
| Criteria | CR | PR | SD | PD |
|---|---|---|---|---|
| Enhancing disease | None | ≥50% ↓ | <50% ↓ but <25% ↑ | ≥25% ↑ a |
| T2/FLAIR | Stable or ↓ | Stable or ↓ | Stable or ↓ | ↑ a |
| New lesion | None | None | None | Present a |
| Corticosteroids | None | Stable or ↓ | Stable or ↓ | NA b |
| Clinical status | Stable or ↑ | Stable or ↑ | Stable or ↑ | ↓ a |
| Requirement for response | All | All | All | Any a |
a Progression occurs when criterion present.
b Corticosteroid dose increase alone is not considered in determining disease progression when there is no persistent clinical deterioration.
PsP
Because of the possibility of PsP, the RANO recommendations state that patients within 12 weeks of the completion of chemoradiation should be excluded from clinical trials for recurrent glioma unless TEP is shown as new enhancement outside of the radiation field or there is unequivocal evidence of tumor at tissue sampling (see Table 2 ). If patients remain clinically stable and/or are thought to have PsP based on functional imaging, then therapy should remain unchanged. Pope and Hessel raised concern that these new recommendations may exclude the most malignant tumors that progress rapidly, and that, because these patients were not excluded from many prior clinical trials, a bias may be introduced when the therapeutic efficacy of a new drug is compared with historical controls. Furthermore, the RANO guidelines do not account for the possibility of PsP occurring at time later than 3 months. The risk of excluding TEP must be considered against the risk of including PsP in clinical trials.
PsR
Given the possibility of PsR, the RANO Working Group has issued new recommendations for patients with recurrent glioblastoma on antiangiogenic therapy (see Tables 2–4 ). These recommendations now state that disease progression can be manifested by a significant increase in the amount of nonenhancing T2W/FLAIR signal while the patient is on stable/increasing corticosteroid dose compared with the baseline scan or best response after the start of therapy. For this reason, the RANO criteria have been referred to as MacDonald plus FLAIR. However, these criteria contain some ambiguity because the definition of a significant increase in T2W/FLAIR signal was not explicitly defined. Another difficulty rests in the recommendation that nonenhancing T2/FLAIR tumor progression must be differentiated from radiation effects, demyelination, infection, decreased corticosteroid dosing, ischemic injury, other treatment effects, and seizures. Imaging findings that would suggest nonenhancing tumor include mass effect, infiltration of the cortical ribbon, and location beyond the radiation field. The RANO criteria also recommend retrospective backdating of the time when nonenhancing progression was first suspected, and although this could increase sensitivity for progression, there is concern that comparison with historical controls could again be difficult. Discordant interpretations can be common in antiangiogenic therapy drug trials and inconsistent progression dating is an issue. Pope and Hessel advocate that making note of suspicious regions of possible nonenhancing progression that are retrospectively confirmed as tumor progression could decrease the discrepancy or adjudication rate.
Other concerns regarding the RANO criteria include issues relating to corticosteroid dosage and lack of validated measures of neurologic function. Regarding corticosteroid dosing, what qualifies as a significant change in steroid dose, over what time period before imaging should steroid status be considered relevant, and whether total daily dose or average daily dose should be considered are also unclear. Both the MacDonald and RANO criteria also consider neurologic function in their assessment criteria. However, a precise definition currently cannot be provided given the lack of validated measures of neurologic function. In RANO, whether a patient is suffering from neurologic deterioration is left to the discretion of the treating physician. They do recommend consideration of a decrease in Karnofsky performance score, Eastern Cooperative Oncology Group performance status or World Health Organization performance score to determine clinical decline.
Surgically delivered therapies
The Surgery Working Group of RANO recently proposed new guidelines regarding response/progression measures following surgically delivered therapies. A summary of their recommendations is given in Box 1 .
- 1.
Imaging after surgery for both HGG and LGG. Because improved outcomes are seen following maximal resection of tumor, recommendations have been set forth regarding the timing of baseline postoperative contrast-enhanced MR imaging to better assess completeness of resection. As was stated by Wen and colleagues, the recent guidelines stressed that, for HGGs, baseline postoperative MR imaging should occur ideally within 24 to 48 hours after surgery, and no later than 72 hours after surgery, because increased enhancement can develop in the wall of the resection cavity 48 to 72 hours after surgery. This postsurgical enhancement can be mistaken for residual or new enhancing tumor. LGGs typically do not show significant contrast enhancement and so a delay of up to 12 weeks for postoperative MR imaging may be needed to differentiate nonenhancing tumor from edema. This study should be compared with the appearance of T2/FLAIR hyperintensity on the preoperative MR imaging. For both HGG and LGGs, intraoperative assessment of completeness of resection is thought to be an unreliable measure compared with postoperative MR imaging. The importance of DWI to document possible perioperative ischemia caused by microvascular compromise at the surgical resection margins was emphasized. These regions may go on to show contrast enhancement on follow-up imaging that could be misinterpreted as recurrent tumor, and so comparison with the postoperative DWI images may be critical.
- 2.
Updated terminology for completeness of surgical resection. Traditional terminology to describe completeness of surgical resection included descriptors such as gross total resection, near-total resection, subtotal resection, and partial resection. These terms were thought to be subjectively determined and inconsistently used depending on glioma grade, so a set of alternative terms has been suggested. This updated terminology may allow improved design of prospective clinical trials that use a specific extent of tumor debulking for entry and for retrospective studies examining impacts of surgical resection on clinical outcome (see Box 1 ).
- 3.
To determine disease progression, completeness of surgical resection and use of local therapies, if applicable, should be taken into consideration. Certain local therapies such as chemotherapy wafers, immunotoxins, or gene therapies given by direct brain delivery, focal irradiation with brachytherapy and stereotactic radiosurgery, and immunotherapies can result in increased contrast enhancement and may simulate recurrent tumor on conventional MR imaging. Because of the nonspecific findings that local therapies can produce, radiographic progression is discouraged as a primary end point in trials using these agents. Although functional imaging techniques may hold promise to distinguish treatment effects from recurrent tumor, none are yet clinically validated so follow-up conventional imaging and clinical evaluation are recommended. Although biopsy remains an option, there is a concern regarding its ability to determine prognosis in treated glioblastoma. However, tissue sampling may be needed if a clinical trial uses an entry criterion or end point of tumor progression.
- 4.
Clinical trials should allow retrospective evaluation of disease progression. Given that there are no validated imaging methods to distinguish tumor progression from treatment effects (ie, PsP), serial follow-up conventional MR imaging should be allowed to determine whether there is disease progression. An indeterminate designation may be used if tumor progression is suspected but treatment effects still cannot be excluded. If it is borne out that the patient does have tumor progression, then the date of an indeterminate designation is deemed to be the time of true progression.
- 5.
Use of blinded central review in clinical trials. PFS is often used as a surrogate for overall survival (OS) in clinical trials. PFS has the advantage that it can shorten trial duration and is not affected by subsequent salvage treatment. However, its reliance on subjective interpretation of MR imaging studies can be problematic, whereas OS can be assessed objectively. The incorporation of a blinded central review of MR imaging studies into the design of a clinical trial should be considered to address this issue.
- 6.
Volumetric assessment of tumor size and response. Given concerns about current nonvolumetric measuring techniques such as poor accuracy and reliability, lack of comparability between studies given different slice positioning, and difficulty in documenting how measurements were made, consideration should be given to the use of volumetric analysis of whole tumor volumes. There is preliminary evidence to suggest that volumetric analysis is effective and this may become realized as the requisite software becomes more widely available. Volumetric techniques may then be applied toward routine assessment of residual enhancing or nonenhancing tumor volume.
- 1.
Imaging after surgery for HGG and LGG
- a.
Should be performed within 72 hours after surgery to determine extent of resection of enhancing tumors (eg, HGG)
- b.
For nonenhancing tumors (eg, LGGs), final determination of extent of resection may require a delay of up to 12 weeks to allow for edema resolution
- c.
Diffusion-weighted imaging (DWI) should be used to determine regions of perioperative ischemia that could subsequently develop nonspecific areas of enhancement
- a.
- 2.
Updated terminology for completeness of surgical resection
- a.
When enhancing tumor is present, removal of all enhancing tissue should be called complete resection of enhancing tumor rather than gross total resection
- b.
Resection of all enhancing tumor (if present) and all nonenhancing tumor tissue (ie, T2/FLAIR hyperintensity) should be called complete resection of detectable tumor
- c.
Partial resections can also be referred to as partial resection of enhancing tumor or partial resection of detectable tumor
- a.
- 3.
To determine disease progression, completeness of surgical resection and use of local therapies, if applicable, should be taken into consideration
- 4.
Clinical trials should allow retrospective evaluation of disease progression
- 5.
Blinded central review should be considered for use in clinical trials
- 6.
Volumetric assessment of tumor size and response should be used as these techniques become more widely available
LGGs
Although HGGs are the primary focus of this article, a brief overview of response criteria for LGGs is also given. Recent RANO guidelines have also been published regarding assessment criteria in trials of LGG ( Table 5 ). The MacDonald criteria are not well suited for use with LGGs because these tumors generally show little to no enhancement. Although LGGs show a less aggressive clinical course than HGGs, most ultimately relapse as HGG, with poor outcome. Conventional contrast-enhanced CT or MR imaging seems to be insensitive to detect early malignant degeneration. Low or even absent radiographic responses have been seen in several LGG trials despite clinical benefit, including a reduction in seizures and prolonged disease control. The posttherapeutic imaging evaluation of LGG is difficult because T2-weighted signal abnormalities following successful therapy cannot be differentiated from tumor. As a result, the category of minor response has been created. Further confusing evaluation is the possibility of radiation-induced leukoencephalopathy, which also shows T2-weighted signal abnormalities. The use of PFS as primary end point in clinical trials is problematic in LGG because of the slow growth rate of LGG and the rare radiological true responses despite favorable response to therapy. At the same time, the use of OS as an end point presents logistical challenges and is also prone to the effect of other noninvestigational salvage treatment at recurrence. Regardless of whether PFS or OS is used as a primary end point, RANO also recommends that ancillary measures such as measures of cognition, seizure activity, symptom severity and burden, quality of life, and neurologic deterioration be considered. Response to radiotherapy should be performed with MR imaging 3 to 4 months following the end of radiotherapy given the possibility of PsP in LGG.
| Response | Criteria |
|---|---|
| Complete response | All of the following are required compared with the baseline scan: Complete disappearance of the lesion on T2/FLAIR imaging (if enhancement had been present, it must have completely resolved) No new lesions, no new T2 or FLAIR abnormalities apart from those consistent with radiation effects, and no new or increased enhancement Patients must not be taking corticosteroids or only on physiologic replacement doses Patients should be stable or improved clinically |
| Partial response | All of the following are required compared with the baseline scan: ≥50% decrease in the product of perpendicular diameters of the lesion on T2/FLAIR imaging sustained for a minimum of 4 wk compared with baseline No new lesions, no new T2 or FLAIR abnormalities apart from those consistent with radiation effects, and no new or increased enhancement Patients should be on a corticosteroid dose that is not greater than the dose at time of baseline scan, and should be stable or improved clinically |
| Minor response | Requires the following criteria compared with baseline: Decrease of the area of nonenhancing lesion on T2/FLAIR imaging between 25% and 50% compared with baseline No new lesions, no new T2/FLAIR abnormalities apart from those consistent with radiation effect, and no new or increased enhancement Patients should be on a corticosteroid dose that should not be greater than the dose at time of baseline scan, and should be stable or improved clinically |
| Stable disease | If the criteria do not qualify for complete, partial, or minor response or progression, then stable disease is present. It requires: Stable area of nonenhancing abnormalities on T2/FLAIR imaging No new lesions, no new T2/FLAIR abnormalities apart from those consistent with radiation effect, and no new or increased enhancement Patients should be on a corticosteroid dose that should not be greater than the dose at time of baseline scan, and should be stable or improved clinically |
| Progression | Defined by any of the following: Development of new lesions or increased enhancement (imaging evidence of malignant transformation) A 25% increase of the T2/FLAIR nonenhancing lesion on stable or increasing doses of corticosteroids compared with baseline scan or best response after initiation of therapy, not caused by radiation effect or comorbid events Clear clinical deterioration not from causes other than the tumor, or decrease in corticosteroid dose Failure to return for follow-up evaluation because of deteriorating condition or death, unless caused by documented nonrelated causes |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree