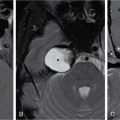
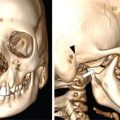
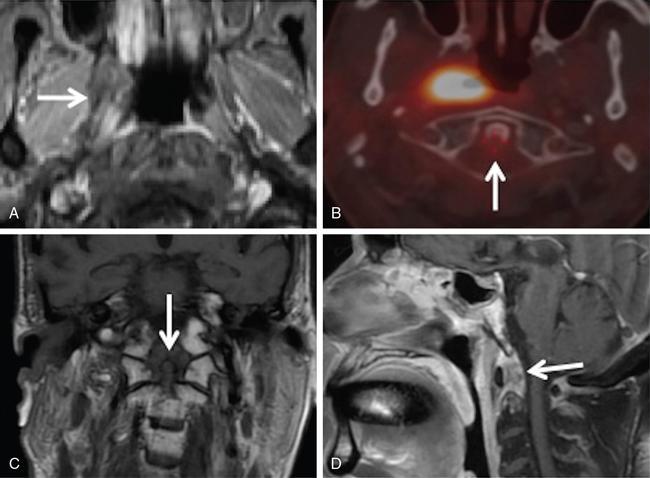
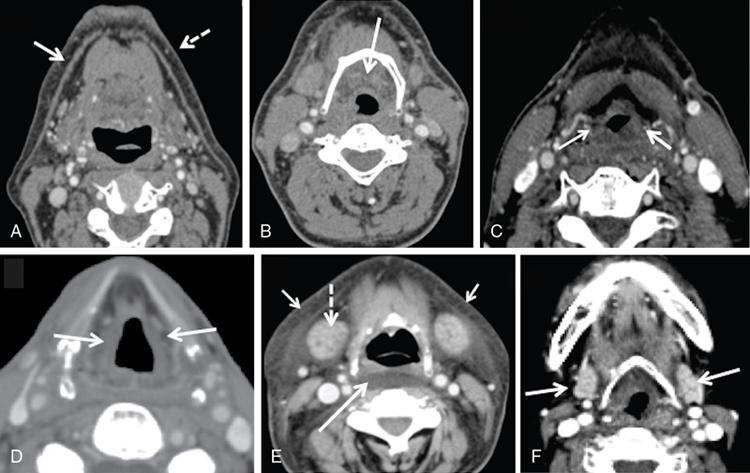
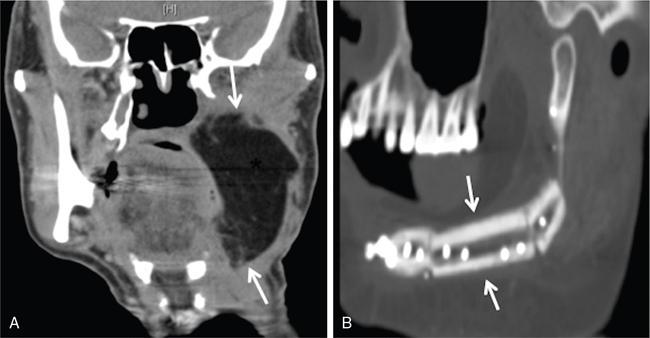
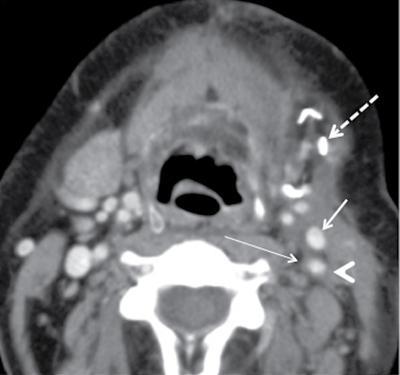
Supreeta Arya, Anisha Gehani, Sanjay Jain, Saugata Sen The incidence of head and neck cancers in India is as high as 57.5% of the global burden and accounts for nearly 30% of all cancers, more so in males of which squamous cell carcinoma forms the bulk. The management of head and neck squamous carcinoma (HNSCC) may require multidisciplinary treatment that includes surgical resection, reconstruction, radiotherapy and chemotherapy. Posttreatment imaging appearances can be perplexing due to anatomical distortion as a result of surgery, changes due to chemo radiotherapy as well as posttreatment complications. The radiologist needs to be familiar with these changes and the complications in order to differentiate these from residual disease or early recurrence so that timely salvage treatment can be planned for the patient. This chapter will familiarize the radiologist with the following: Clinical examination and endoscopy can directly visualize recurrent mucosal tumours in the aero-digestive tract. However, imaging is essential to visualize deeper lesions/lesions with submucosal spread. Imaging also identifies lesions not amenable to visual inspection such as when trismus is present. The role of imaging is not only in diagnosis but in staging, guiding biopsies and treatment planning. Finally, imaging is an important tool in posttreatment surveillance. The various modalities for imaging evaluation of head and neck cancers are ultrasonography (US), Computed Tomography (CT), Magnetic Resonance (MR) imaging and dual-modality imaging with fluoro-2-deoxy-d-glucose (FDG) positron emission tomography (PET) with CT (FDG PETCT). The need for posttreatment imaging evaluation in head and neck cancers arises in the following settings: (1) during treatment for assessing response to induction/first-line chemotherapy (2) in the immediate posttreatment period for detecting response to chemo-radiation, identifying residual disease to plan salvage treatment, identifying cases of early recurrence as well as in assessing posttreatment complications and (3) during follow-up (surveillance) for detecting recurrence and late complications if any. Induction (first line) chemotherapy is sometimes offered prior to definitive treatment with surgery or chemo-radiotherapy. It may be necessary to see the disease response to decide continuation of treatment. However, this is a less frequently used role of posttreatment imaging. Cross-sectional imaging with CT and MRI is used to study this response using RECIST 1.1, which though has limited application in identifying patients with pathological complete response (who might harbour morphological abnormality that might be functionally nonviable). PETCT cannot be used in this early time period (<10 weeks) due to low specificity owing to postchemotherapy inflammatory response. There have been investigational reports for use of CT perfusion and diffusion-weighted MRI (DW MRI) as posttreatment imaging tools for very early prediction of response to induction chemotherapy in head and neck cancers with encouraging results. The exact choice of the imaging method depends on the type of treatment offered and the region of the primary. In laryngeal cancers where either total laryngectomy has been performed or nonsurgical chemoradiation therapy has been offered as well as in naso-oropharyngeal cancers treated with chemoradiation, FDG PETCT can be very useful in identifying loco-regional residual disease and has high sensitivity and negative predictive value (NPV) as shown in a systematic review and metaanalysis. Higher diagnostic accuracy is observed in scans done ≥12 weeks. The highest benefit of PET-CT is at 3 and 6 months, with a negative predictive value of 91% and 98%, respectively. Problems with PETCT are the cost, exposure to radiation, interpretation challenges when the tumour is too small and lower positive predictive value (79%) if performed earlier than 12 weeks due to the false positives. PET-CT done too early can also give rise to false negatives. Cross-sectional imaging with CT/MRI may be cost-effective alternatives and NCCN guidelines recommend a baseline cross-sectional contrast-enhanced CT or MR imaging of the head and neck 3–4 months following treatment of loco-regionally advanced disease. The aim of CT/MR imaging is to identify residual disease to plan salvage therapy as well as to detect treatment complications. A chest CT may or may not be added. In fact, the advantage of CT and MR imaging is that it can be performed even earlier (unlike PETCT), immediately after treatment completion or at 4–8 weeks after treatment. For imaging the posttreatment larynx, MRI is beset with image degradation due to motion artefacts. Hence, except in centres with high expertise in laryngeal MR imaging, contrast-enhanced CT is the preferred cross-sectional imaging method in the posttreatment larynx. However, in patients with salivary gland, sinonasal, nasopharyngeal, oropharyngeal and skull base tumours (those at risk for perineural invasion or dural invasion), contrast-enhanced MRI is the preferred imaging method. Perineural spread is best assessed with postgadolinium T1W fat-suppressed images. In the posttreatment oral cavity with soft tissue reconstruction, recurrences within the flap may be missed on CT and are better assessed by contrast-enhanced MRI. MDCT though provides better evaluation of cortical bone such as mandible or maxilla and small recurrences eroding the cut end of hemimandibulectomy site are well seen. MDCT also has the advantage of rapid acquisition, hence proving helpful in head and neck cancer patients experiencing difficulty with breathing, swallowing secretions and lying flat. PETCT is also an excellent tool to assess small soft tissue recurrences in the flap and in equivocal cases on cross-sectional anatomic imaging (Fig. 3.39.1). At times, a combination of CT and MRI may be useful, including advanced imaging techniques. The use of DW MRI increases the sensitivity for recurrent head and neck cancer. US too has a role in posttreatment evaluation. It is usually reserved for assessing the neck and for targeted sampling of suspicious nodes; however, the results may be inconclusive whilst sampling nodes in the early weeks after radiation therapy. Hence, response assessment is often based on morphological changes seen on serial US scans. Nodes that show reduction in size or are unchanged in size and appearance on serial US studies may suggest response. More recently, in an attempt to make interpretation of the posttreatment neck more objective, the American college of Radiology (ACR) has introduced the Neck Imaging Reporting and Data system (NI-RADS) for describing both the primary and neck. In this system, scores are assigned (NI-RADS 0–4) to various categories of suspicion of recurrent disease using cross-sectional imaging and/or PETCT. NI-RADS will be described in a later section. Head and neck cancers pose a challenge in the posttreatment surveillance setting. Anatomical distortion and fibrosis following surgery and radiotherapy lead to loss of symmetry that has a potential to mimick recurrence. Clinically, occult recurrences or residual tumour may also be mistakenly reported as posttreatment changes. Currently, there is no consensus regarding the frequency or type of posttreatment imaging in the surveillance period in an asymptomatic patient. Various institutions have different protocols in place for the timing and type of surveillance imaging. CT, MRI, US and PET CT have their own strengths and limitations for posttreatment surveillance. PETCT is particularly useful for both evaluation and staging of suspected recurrence. Multiple studies have established the role of PETCT in head and neck cancer surveillance. A systematic review and metaanalysis of studies involving 2335 patients assessing the diagnostic performance of PETCT in response assessment and surveillance imaging of HNSCC patients reported a high sensitivity (85%), specificity (89%), NPV (94%), but a suboptimal PPV (<60%) both for the primary site and cervical nodal recurrence. The high sensitivity of PETCT in detecting recurrence was noted at 3 months, 4–12 months and even >12 months. Also in established recurrent head and neck cancer, PETCT is recommended before planning salvage treatment, as it has high sensitivity and accuracy for screening distant metastases and can even detect an unknown new primary (Fig. 3.39.2). However asymptomatic patients with a negative 3 month PETCT had very low incidence of a positive PETCT at 12 or 24 months. The incidence of a recurrent or second primary disease was 10% at 12 months and approximately 5% at 24 months. Hence, the benefit of subsequent surveillance with PETCT in asymptomatic patients is limited and debated. However, imaging is mandatory in the patient that develops worrisome symptoms or signs during the surveillance period. Additionally, routine imaging at annual clinical surveillance may be required for areas not amenable to clinical inspection. In suspected recurrent nasopharyngeal cancer, PETCT and contrast-enhanced MRI are complementary. Whilst PETCT is superior for distinguishing recurrent mucosal disease from scar tissue, MRI is very useful for detecting marrow metastases, skull base invasion and perineural spread (Fig. 3.39.3). Recurrence of HNSCC typically occurs between 1 and 3 years, and has to be differentiated from osteoradionecrosis (ORN) which is also seen during the same time period. CT is the best method to demonstrate ORN, whilst the increased uptake seen on PETCT in ORN might be mistaken for recurrent disease. US has a role for nodal surveillance and for needling suspicious nodes. However, it has to be kept in mind that whilst the accuracy of fine needle aspiration in enlarged nodes with suspicious US features is high, the accuracy is significantly lower in subcentimetre sized nonpalpable nodes that may require needling due to suspicious morphological features. The treatment of head and neck cancers is stage dependent. Early stage disease (Stage I and II) are managed with single modality therapy, surgery or radiotherapy. Locally advanced head and neck cancers (Stage III and IV) without distant metastases are treated with multimodality treatment that includes a combination of surgery, radiotherapy and chemotherapy for addressing the primary tumour and the neck node metastases. Following curative surgery for advanced primary disease, reconstructive surgeries may be performed to restore function and cosmesis. The neck is addressed surgically by various kinds of neck dissection or could be treated with radiation therapy. Advanced cancers of the oral cavity, sinonasal region and salivary glands are principally treated with surgery followed by adjuvant radiotherapy/chemoradiation. Advanced nasopharyngeal, oropharyngeal, laryngeal and hypopharyngeal cancers are usually treated with chemoradiation. Head and neck cancers have a high possibility of cure if diagnosed in early stages, but two-thirds of the patients are diagnosed at an advanced locoregional stage (stages III and IV, without metastasis). HNSCCs are treated with either external beam radiation therapy (EBRT) or brachytherapy. Brachytherapy involves implanting the radiation source temporarily or permanently at the site of disease such as iodine seeds or iridium. EBRT which is more commonly used is delivered from a source external to the patient using electron beam, photon beam or proton beam. The treatment regimen involves delivering daily doses of 65–70 Gy radiation, for 6–7 weeks. Photon beam EBRT can be delivered using the three-dimensional conformal radiation therapy technique or using the intensity-modulated radiation therapy (IMRT) method. The latter is the preferred method in the last decade as it has the advantage of better coverage of the tumour, whilst sparing organs at risk such as the parotid glands and the pharynx. Proton beam radiation therapy is used in treating skull base chordomas and sinonasal tumours. Certain changes are seen in the head and neck region after radiation therapy and these are divided into early reactions seen in the first 3 months after completion of radiation therapy and late reactions seen after 3 months. The early reactions are encountered often and are reversible and include oral mucositis and skin desquamation. Xerostomia, dental caries, dysphagia, osteoradionecrosis, radiation vasculopathy and radiation-induced neoplasms are late reactions seen after radiation therapy. The severity of the reactions depends on the dose of radiation and the technique used as well as the radiosensitivity of the patient. Some of these reactions are visible on CT and MR imaging. The early and late changes seen on imaging are listed in Table 3.39.1 and depicted in Fig. 3.39.4. Following radiotherapy, an acute inflammatory reaction is seen within the deep tissues after 2 weeks with resultant interstitial oedema. This is seen on imaging as reticulation of subcutaneous and deep fat and oedema in the deep neck spaces such as the carotid sheath and retropharyngeal space. Mucositis and submucosal oedema result in mucosal contrast enhancement with thickening of the pharyngeal and laryngeal structures. Subsequently, there is connective tissue thickening, fibrosis and reduction of interstitial oedema. The salivary glands and thyroid initially appear oedematous, eventually being replaced by fatty atrophy. It is important to note that the expected tissue changes after radiation therapy appear symmetrical, unless the neck is irradiated using asymmetric radiation portal. Surgery for HNSCC involves the following: Excision of lesions may result in large defects which are required to be closed using flaps so as to maintain cosmesis and function. Different types of flap reconstruction techniques have evolved over time. Flaps can be divided into simple or composite depending on the composition of the tissues. Simple flaps contain one type of tissue component like skin and subcutaneous tissue. Composite flaps contain more than one type of tissue (fat, cutaneous, muscle, osseous tissue) such as the myocutaneous flap. Based on the reconstructive technique, flaps can be local flap, free flap or pedicle flaps. Local flaps involve simply repositioning of adjacent tissues and are less frequently used. More commonly used flaps in the head and neck region are pedicle flaps (simple rotation of adjacent tissues preserving inherent vascular pedicle) and free flaps (flaps that are raised along with their local vascular supply and reconstructed at the surgical site with microvascular anastomosis techniques). These flaps are often composite flaps. The pectoralis major myocutaneous (PMMC) flap is the most common pedicle flap used in the head and neck region, whilst the free flaps used are rectus abdominus myocutaneous free flap, antero-lateral thigh flap, radial forearm free flap, fibular free flap and jejunal free flap. In imaging studies, flaps appear as bulky soft tissue structures, showing the characteristics of predominantly muscle in a myocutaneous flap; gradually, denervation atrophy occurs, causing volume loss and fatty replacement of the muscle (Fig. 3.39.5A). Muscle denervation may be incomplete at the time when imaging is performed. Hence, muscle density fibre-like structures seen within the flap should not be confused with tumour recurrence. Another important feature to recognize is the sharp boundaries between the flap and adjacent structures that indicate benignancy (Fig. 3.39.5A). Fibular free flap is seen in Fig. 3.39.5B. HNSCC at each site may spread to regional nodes. Complete oncological treatment requires curative resection of the primary lesion along with complete removal of the nodes to which the primary disease may have metastasized to. Adequate treatment of the neck requires unilateral or bilateral standardized neck dissections where the lymph nodes and surrounding tissue in a predefined anatomic area are removed. There are three kinds of neck dissections: radical neck dissection, modified radical neck dissection and the selective neck dissection. Radical neck dissection (RND) involves removal of ipsilateral nodes (level I–V) along with ipsilateral nonlymphatic structures such as internal jugular vein, submandibular gland, spinal accessory nerve and sternocleidomastoid muscle. Modified radical neck dissection (MRND) also involves removal of ipsilateral level I–V nodes, but preserves one or more of the above mentioned nonlymphatic structures, resulting in less deformity and less morbidity (Fig. 3.39.6). Selective neck dissection involves removal of nodes of a particular station, such as supraomohyoid type (I–III) and anterior nodal compartment (VI and VII), the latter commonly employed for thyroid malignancies. Selective neck dissection preserves cosmesis and functional structures. Whilst RND and MRND are easy to recognize on CT or MR imaging due to absence of the removed nonlymphatic structures, it may not always be possible to identify performed selective neck dissection on imaging if the nonlymphatic structures are preserved. Concentric soft tissue cuffing surrounding the carotid sheath is seen typically both after RND and MRND (Fig. 3.39.6).
3.39: Posttreatment imaging in head and neck cancer
Introduction
Learning objectives
Imaging methods
Response assessment during treatment
Immediate posttreatment (up to 6 months after treatment completion)
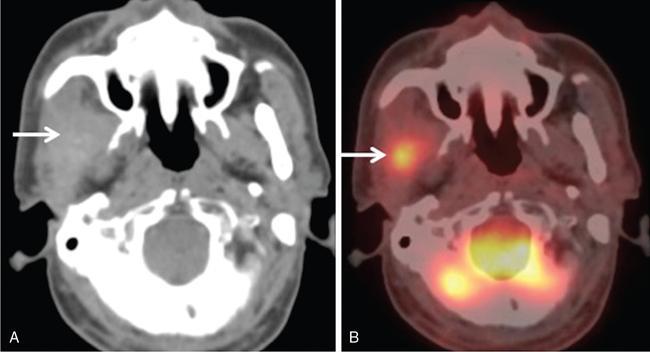
Surveillance (>6 months after treatment completion)
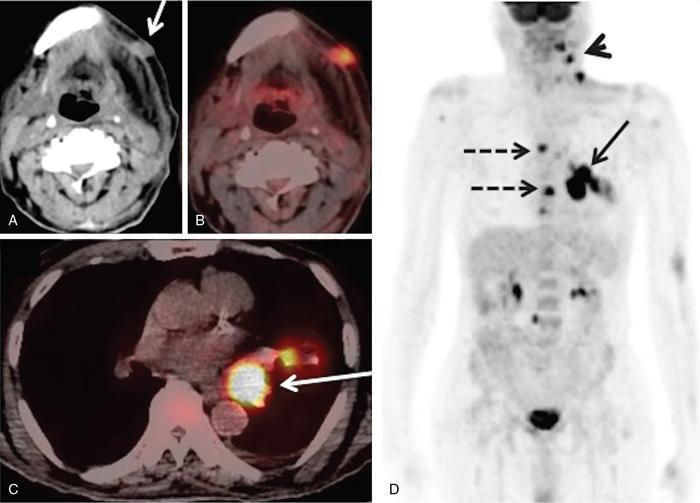
Pearls
Treatment methods and expected posttreatment changes
Radiation therapy
Expected changes postradiation therapy
Early (<3 Months)
Late (≥3 Months)
Thickening of skin and platysma
Thickened skin and platysma
Reticulation of subcutaneous fat
Increased enhancement of the major salivary glands
Atrophy of salivary glands
Oedema and fluid in the retropharyngeal space
Thickening and increased enhancement of the pharyngeal walls
Thickened pharyngeal constrictor muscles
Thickening of laryngeal structures (epiglottis, aryepiglottic folds, preepiglottic and paraglottic spaces, true and false cords)
Surgery
Surgical reconstruction techniques
Expected changes after reconstructive surgery
Neck dissection
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree