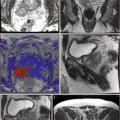
Fig. 5.1
Schema of normal intraprostatic and intracapsular blood supply. 1 Internal iliac artery, 2 prostatic vesical artery, 3 inferior vesical artery, 4 prostatic artery, 5 capsular artery, 6 parenchymal artery, 7 intracapsular artery (pedicular origin), 8 intracapsular artery (vesical origin), 9 intracapsular artery (urethral origin)
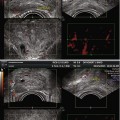
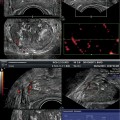
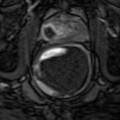
With PDS and 3D PDS, in normal subjects (Fig. 5.2a–c) at the level of the peripheral prostate and at the lateral edges, capsular arteries radially supplying the parenchymal branches were found in all the patients: on coronal section they were symmetrically distributed with a weak Doppler signal. There were no secondary division branches observed at the level of the intra-prostate parenchymal vessels. In 26 of the 41 referring subjects, a 1–2 mm thick posterior peripheral margin was identified that contained vessels of diverse origin: sagittally, medially, and laterally arranged vessels having their origin in the inferior vesical net; others having a periurethral origin; and finally, others originating at the lateral pedicular level. On 3-D PDS reconstruction, this group of vessels forms a marginal system that delimits the posterior surface of the prostate. In the normal state, there were no apparent anastomoses visualized on power Doppler sonography appearing between the vessels forming the marginal system and the intraparenchymal or extra-prostate vessels. Near the posterolateral border, identification of neurovascular bundles in this referring group was suggested on coronal scans on both sides of the prostate-like vessels giving branches to the posterior peripheral margin.
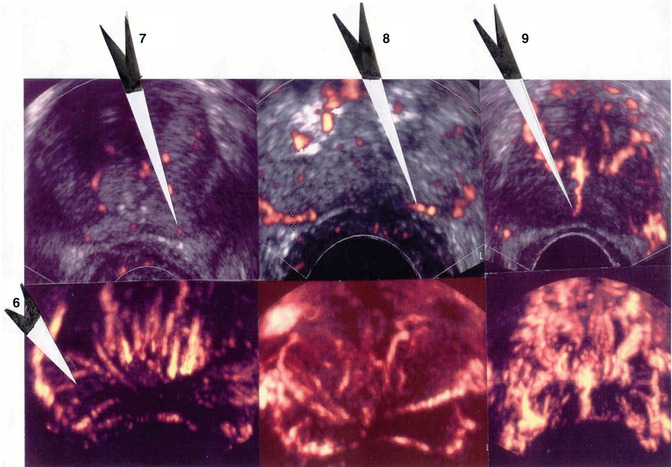

Fig. 5.2
(a) Axial section: Visualization of three vessels in posterior peripheral margin on axial section and placed symmetrically on 2D imaging. The capsule is raised in 3D by its vessels. (b) Axial section: posterior peripheral vessels of left and right pedicular origin on power Doppler and in 3D PDS. (c) Axial section: Anastomosis between periurethral and posterior peripheral vessels
5.3 Equipment and Techniques
No specific patient preparation is required. The patient is placed in the left lateral decubitus position. A digital rectal examination is recommended prior to probe insertion to rule out any obstructing pathology and also to allow the examiner to evaluate the prostate by digital examination. The probe is covered with a condom into which coupling gel has been placed and the probe lubricated and gently inserted into the rectal canal.
5.3.1 Sonography Technique and Power Doppler Mode
Examination of the prostate by ultrasound requires a high-frequency (7.5–10 MHz) end-fire or biplane transrectal transducer. We used Philips HDI 5000 power Doppler sonography instruments (Philips Ultrasound, Bothell, WA, USA) coupled with a C9-5 ICT endocavity probe and Toshiba Aplio MX (Toshiba MS, Nasu, Japan) coupled with a biplane PVT-770RT endocavity probe (5–10 MHz). Examination of the prostate by grayscale imaging is first performed and the length, width, and height of the gland measured. The prostatic volume is calculated based on the formula for a prolate ellipsoid (length × width × height × 0.523); this allows correlation of the measured PSA with a predicted PSA based on gland volume.
As a complement to the endorectal sonography, an exploration with the power Doppler mode was carried out in all patients. The Doppler increase was optimized in each patient so that there was no background noise at about 80 %. We privileged a low PRF that was adjusted to 500 Hz in standard value: the filtration and persistence were preset at their maximum level in order to limit the movement artifacts.
A weak compression with the end of the probe was systematically carried out in case of a hypoechogenic area in the search for a modification of its echostructure. The mean time for a Doppler sonography of the prostate was about 15 min.
Three-dimensional reconstruction with power Doppler of intraprostatic blood flow imaging (3D PDS) was performed for a better understanding of the prostate perfusion. 3D PDS was obtained with the capture of 11 power Doppler US images after scanning a suspicious volume. Then all these images were added in a three-dimensional reconstruction allowing us to make an angular movement of 15°. On the final image, only power Doppler acquisitions were present without grayscale data.
5.4 Value of Transrectal Power Doppler Sonography in Suspect Subjects of Cancer of the Prostate (PSA level > 4ng/ml or Abnormal DRE) Before a First Series of Biopsy
Prostate cancer is generally hypervascular when compared to normal prostatic tissue, and this is manifested as increased color encoding at sensitive instrument settings. 3D PDS looks for area or intraprostatic volume with an increase in the number of detectable vessels or asymmetry in the distribution of blood flow in each investigated case. A vascular density in the suspected area or volume was noted.
The criteria of analysis were: increase in number of intra-lesion vessels, disoriented vessels or verticalized vessels in peripheral gland, asymmetrical blood flow, mass effect on the intra-prostate perilesional vessels and vessels in peripheral margins (Fig. 5.3a, b).
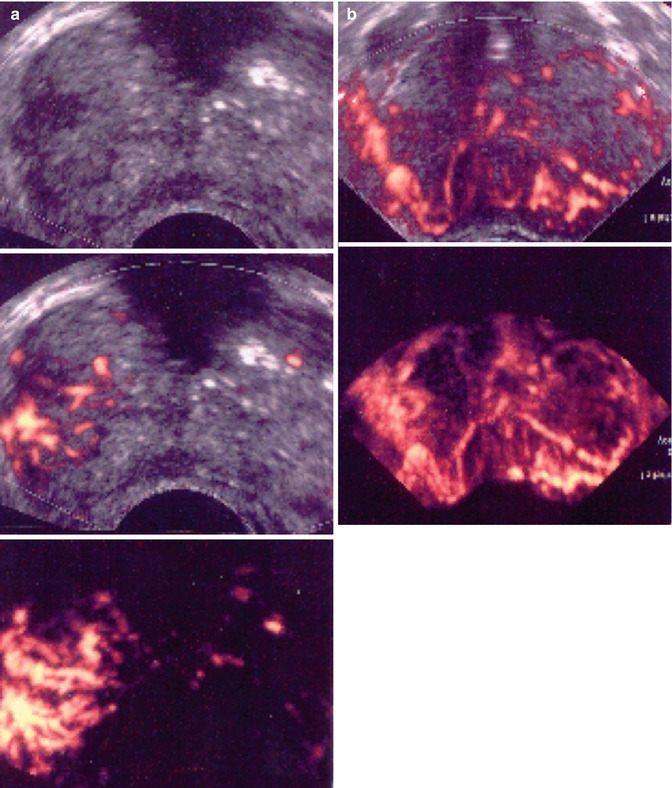

Fig. 5.3
(a) Prostate cancer with 3D PDS vascular asymmetry or irregular vessels in any part of the prostate. (b) Prostate cancer with 3D PDS vertically or disoriented parenchymal vessels
5.4.1 Ultrasound Semiology and Power Doppler (Sauvain et al. 2003, 2006)
In B mode, the peripheral area was in the normal state homogenous and more echogenic than the transition area.
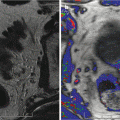
The suspect ultrasonographic anomalies in the peripheral prostate area may be qualified as hypoechogenic, weakly hypoechogenic or subtle (Figs. 5.4a–c and 5.5a–c), and heterogenic. The power Doppler mode searched for the presence of tumor vessels in a suspect lesion in the B-mode sonography. A visible lesion in B mode was said to be hypervascularized if it included one or several vessels. The lesion was qualified as focal if it was less than 5 mm. However, this lesion was qualified as nodular if it was over 5 mm and confined to a single sextant, comprising vessels remaining strictly intralesional. If the zone hypoechogenic area involved three sextants or more and/or if the vessels extended beyond the hypoechogenic area, the lesion was qualified as infiltrating (Fig. 5.4). If the lesion was nodular or focal and associated with outer seats without real contiguity, the lesion was qualified as multifocal.
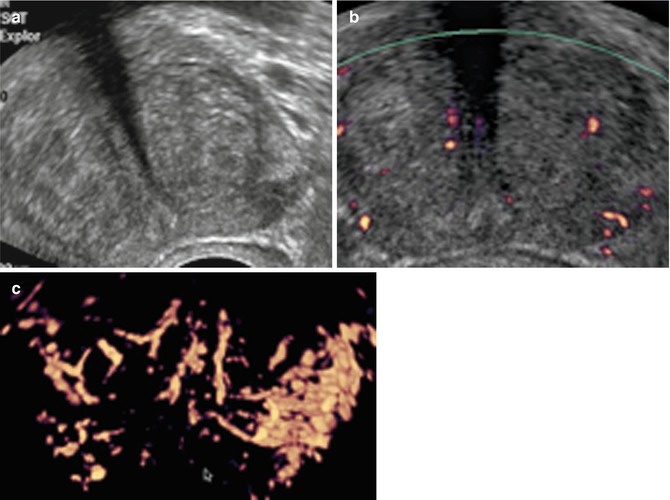
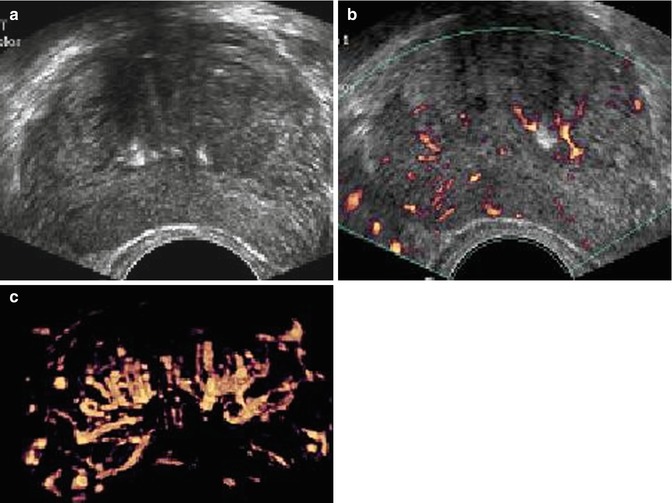

Fig. 5.4
Subtle hypoechoic and hypervascular lesion. (a) B mode: isoechoic lesion. (b) Increase in vessel number in left sextants. (c) 3D vascular reconstruction (PSA 10 ng/ml, 3 positive biopsies in left sextant, Gleason score 7(4 + 3), extraprostatic extension)

Fig. 5.5
Isoechoic and hypervascular lesion. (a) B mode: isoechoic lesion. (b) Increase in vessel number in right sextants. (c) 3D vascular reconstruction (PSA 10 ng/ml, 3 positive biopsies in right sextant, Gleason score 6, no extraprostatic extension)
A minority of cribriform carcinomas can demonstrate punctate calcifications.
5.4.2 Four Classes for the Different Vascular Tumor Types (Sauvain et al. 2003)
The PDS results can be rated from 1 to 4 classes:
1.
Normal
2.
Slightly hypoechogenic avascular lesion in which the hypoechogenicity disappears after slight compression with the probe (Fig. 5.6)
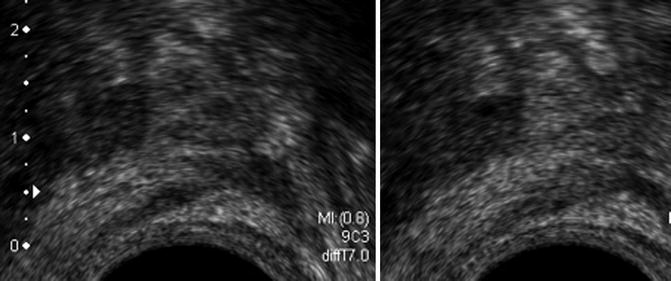

Fig. 5.6
Class 2. (a) (Left) Low hypoechoic lesion of peripheral prostate without vascular anomaly that disappears under compression. (b) (Right) PSA 6.2 ng/ml, negative biopsies
3.
Hypoechogenic avascular lesion (Fig. 5.7)
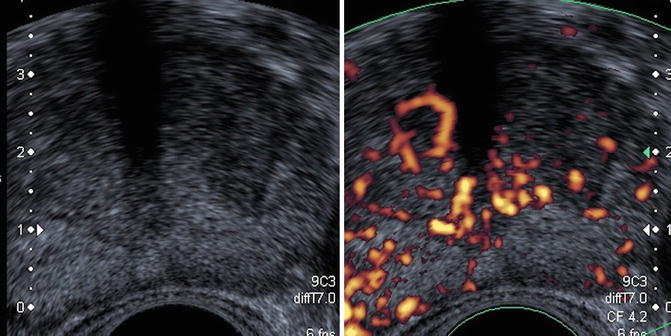

Fig. 5.7
Low hypoechoic lesion of peripheral prostate without anomaly (a) that does not disappear under compression (b)
4.
Weakly hypoechogenic hypervascularized lesion and isolated hypervascular lesion (Fig. 5.8) or hypervascularized hypoechogenic lesion (Fig. 5.9)
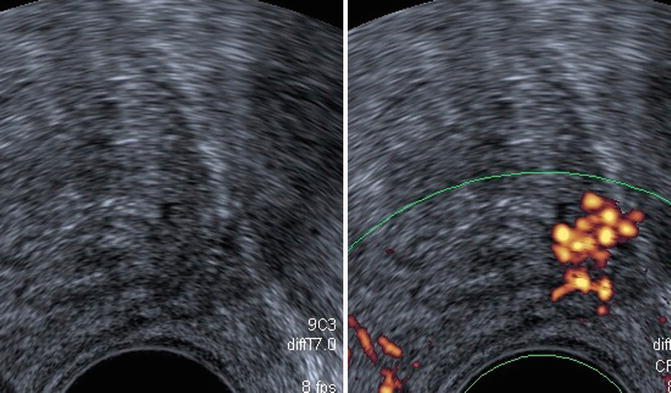
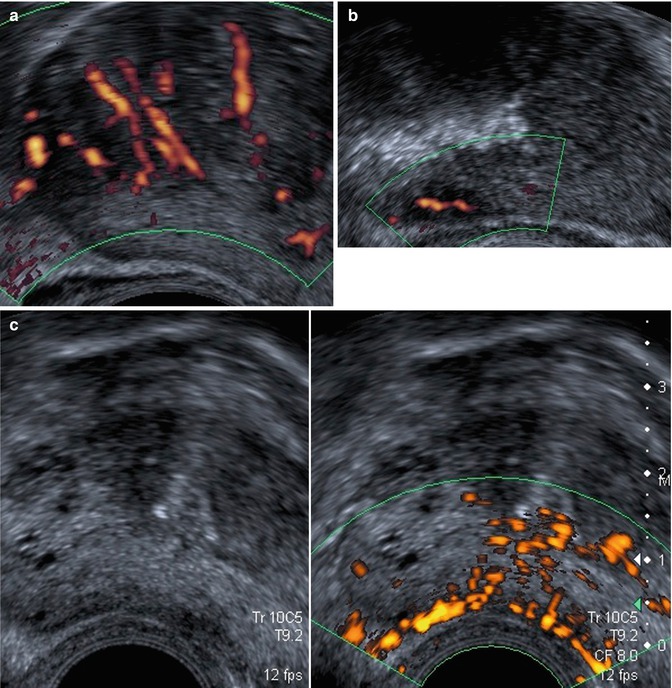

Fig. 5.8
Class 4. Low hypoechoic vascular lesion of peripheral prostate: 120 g, normal DRE, PSA: 9.6 ng/ml, Gleason 7 (3 + 4) score

Fig. 5.9
Class 4. Non-palpable hypoechogenic and hypervascular lesions: (a) PSA 4.5 ng/ml, Gleason score 7 (4 + 3), one positive biopsy. (b) PSA 7.4 ng/ml, Gleason score 6 (3 + 3), two positive biopsies included on invaded greater than 50 %. (c) PSA: 6.2 ng/ml, Gleason score 6 (3 + 3): three positive biopsies
Classes 3 and 4 are considered to be pathological.
5.4.3 PDS and Tumor Location
The base and apical margins are the most important margins as they are the ones most frequently involved by infiltration of the cancer. Apical prostate cancer demands particular attention in terms of whether or not it is really organ confined. Some 80 % of apical prostate cancers are close to the apical capsule, and the cancers here are at risk of capsular penetration along apical nerve branches entering the prostate allowing the cancer to infiltrate (perineural infiltration) along these conduits out of the prostate (Fig. 5.10).
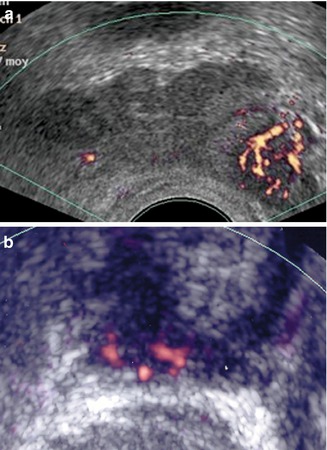

Fig. 5.10
(a) PCa in the left base. (b) Apical prostate cancer
As an aside, this is not to say that all men who have clear base and apical margins to their prostate will remain free from disease forever, as some 10–15 % of men with localized or organ-confined disease will develop spread of their prostate cancer after radical surgical/robotic removal. It is unknown if the manipulation from radical surgery/robotics actually contributes to this problem of cancer spread or whether there are other factors involved.
The “apex” is the most frequent location of PCa, and there is a high false-negative rate from transrectal biopsy. It is difficult to predict apex cancer preoperatively using methods currently available.
Transrectal PDS allows one to localize a lesion in the apex or base area (Fig. 5.11a–c




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree