Group
Characteristic
Patients, (%) (n = 410)
A
(Flu+/Dye+)
381
(92.9 %)
B
(Flu+/Dye-)
18
(4.4 %)
C
(Flu-/Dye+)
0
(0 %)
D
(Flu-/Dye-)
11
(2.7 %)
Table 9.2
Comparison of detection rate (upper) and identified mean numbers (lower) in sentinel lymph nodes in clinical trials
Study | Year | ICG | BD | RI | |||
|---|---|---|---|---|---|---|---|
Tagaya et al. [14] | 2008 | 100 % | (25/25) | 92 % | (23/25) | ||
5.5 | 2.3 | ||||||
Abe et al. [15] | 2011 | 100 % | (128/128) | 66 % | (84/128) | ||
3.1 | 1.0 | ||||||
Hirano et al. [16] | 2012 | 99 % | (107/108) | 93 % | (100/108) | ||
2.2 | 1.6 | ||||||
Sugie et al. [17] | 2013 | 99 % | (98/99) | 78 % | (77/99) | ||
3.4 | 2.4 | ||||||
Hojo et al. [18] | 2010 | 99 % | (140/141) | 92.9 % | (105/113) | 100 % | (28/28) |
3.8 | 1.9 | 2.0 | |||||
Wishart et al. [11] | 2012 | 100 % | (104/104) | 99 % | (103/104) | 91 % | (95/104) |
– | – | – | |||||
Jung et al. [19] | 2014 | 100 % | (43/43) | 91 % | (39/43) | 100 % | (43/43) |
3.4 | – | 2.3 | |||||
9.3.1 Device Used
Fluorescence navigation of lymphatic channels and SLNs is achieved using a near-infrared fluorescence navigation device (Photodynamic Eye (PDE) camera or Photodynamic Eye-neo (PDE-neo) camera, Hamamatsu Photonics K.K., Hamamatsu, Japan).
9.3.2 Use of ICG as a Tracer
Figure 9.1 shows the strong illumination of diluted ICG. The dilution must be considered to be due to a “quenching reaction.” All fluorophores exhibit fluorescence quenching if their concentration is too high, and near-infrared fluorophores are no exception [20]. That is, increasing the concentration actually decreases the fluorescence, so theoretically there would be no benefit to injecting a high concentration for SLN mapping [21]. It has been suggested that the degree of hypersensitivity symptoms resulting from diluted ICG is lower compared to a non-diluted dose; therefore the diluted ICG is also preferable from a safety perspective. In our previous study of 410 patients who received an intraoperative injection of ICG, none of these patients experienced any related adverse reactions or complications [13]. Careful attention to concentration and dilution is a factor that enables improved performance of the technology [22].
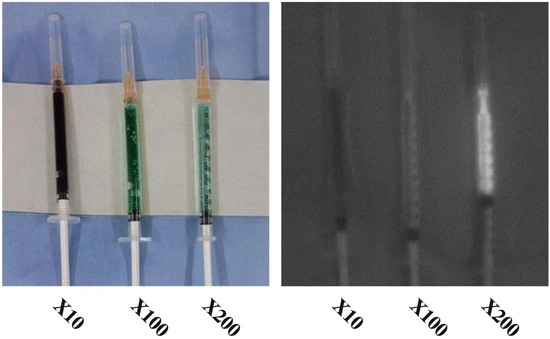

Fig. 9.1
Concentration-dependent quenching. The fluorescence intensity of changed in each sample (indocyanine green) when proportionally diluted with saline
9.3.3 Tracer Preparation and Injection
ICG (25 mg/5 mL, Diagnogreen; Daiichi–Sankyo Co., Ltd., Tokyo, Japan) is diluted 100 times with saline considering “quenching reaction,” resulting in ICG concentration of 0.05 mg/mL (0.064 mM). Then the mixture agents of 1.0 ml (0.05 mg) of diluted ICG and 1.0 ml of blue dye (indigo carmine (4 mg): Daiichi–Sankyo Co., Ltd., Tokyo, Japan) is injected subdermally into the areola. A thin injection needle (thinner than 27 gauge) is used for the purpose of taking pressure. If using ICG alone without using blue dye, 2.0 ml of diluted ICG has been found to be effective with this method.
9.3.4 Surgical Procedures
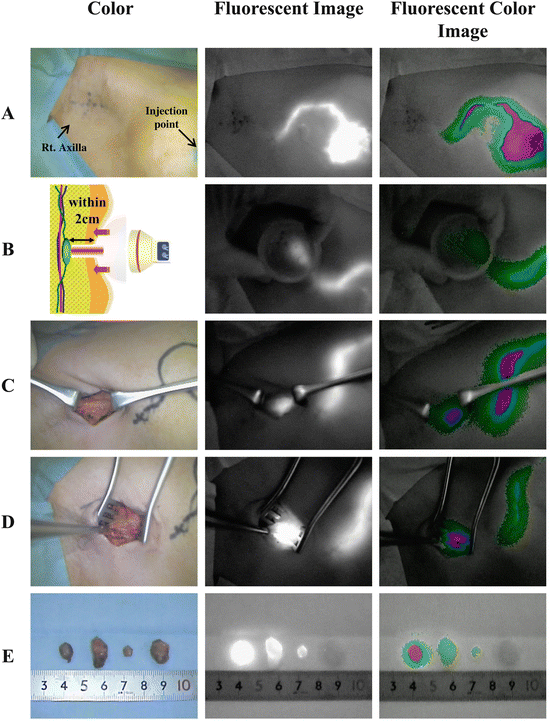
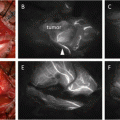
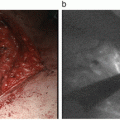
Within a couple of seconds after injection, the illuminated subcutaneous lymphatic channels and veins traveling toward the axilla can be visualized clearly. Gentle massage of the pomphus, which forms due to retention of the tracer, helps the lymphatic drainage. After a few minutes, only illuminated lymphatic channels remain visible (Fig. 9.2A); usually these lymphatic channels converge toward a single duct in the region of the axilla tail, and they can be traced to the axillary region where occasionally nodes can be seen percutaneously. These subcutaneous lymphatic channels are often invisible through the skin beyond the lateral edge of the greater pectoral muscle, where they extend deeper into the axillary space (Fig. 9.2A). To overcome the invisible SLNs from the skin, axillary compression technique was designed [23]. Use of a hemispherical transparent device (Hamamatsu Photonics K.K., Hamamatsu, Japan) enables the easy observation of deeper lymphatic structures. Figure 9.2B (left) depicts the principal of the technique and an actual observation. From the point where the illumination disappears, the chest wall is compressed by the hemispherical transplant device, allowing the observation of the liner fluorescence signals, which are lymphatic channels. Searching the illuminated lymphatic channels, the SLNs appear as round- or oval-shaped illuminated objects with intensity fluorescence signals as shown in Fig. 9.2B (middle). The site of the skin incision can be more precisely localized following this compression method. The major disadvantage of the ICG method compared to the RI method, the low penetration of the signal, is improved using this axillary compression technique. If transcutaneous identification of the SLNs is unsuccessful, the original method, in which a skin incision is made around 2 cm ahead from the vanishing point of fluorescence or at approximately 1–2 cm proximal to the hairline, is used. Once the axillary skin incision on the round- or oval-shaped illumination, following to the superficial connective tissue separation, has been performed. A vague, weak, and ill-defined fluorescence signal can be observed without a transparent device, affecting the fluorescence signal from the SLNs. As shown in Fig. 9.2C, in the combination of ICG and blue dye, a blue-stained structure is not clear, but a vague fluorescence is observed. When the tissue and fascia are dissected and separated toward the fluorescence signal, the intensity of the signal increases as the SLNs are approached. The illuminated fat tissues including SLNs are pulled from the surgical field, and the intensive fluorescence nodes are separated from any surrounding fatty tissue (Fig. 9.2D). The illuminated SLNs, with or without blue dye, are resected under the guidance of an ICG fluorescent image. A wide cut in the axillary fascia and the adequate pulling of the fluorescent fat tissue are effective in avoiding injury of the neighboring blood vessels and nerves. During dissection of the illuminated nodes, ICG sometimes can leak into the surgical field and produce nonspecific fluorescence staining, leading to difficulties in detection of other fluorescence nodes. As a solution, decreasing the excitation light leads to a line or disappearance of the nonspecific fluorescence staining. On the other hand, residual SLNs can remain as round- or oval-shaped illuminations even if the excitation light decreases. Finally, all dissected lymph nodes are investigated by the ICG camera (Fig. 9.2E).