The clinical management of melanoma patients has been rapidly evolving with the introduction of new targeted immuno-oncology (IO) therapeutics. The current diagnostic paradigms for melanoma patients begins with the histopathologic confirmation of melanoma, initial staging of disease burden with imaging and surgical approaches, treatment monitoring during systemic cytotoxic chemotherapy or IO therapeutics, restaging after completion of adjuvant systemic, surgical, and/or external radiation therapy, and the detection of recurrent malignancy/metastatic disease following therapy. New and evolving imaging approaches with positron-emission tomography (PET) imaging technologies, imaging methodologies, image reconstruction, and image analytics will likely continue to improve tumor detection, tumor characterization, and diagnostic confidence, enabling novel precision nuclear medicine practices for managing melanoma patients. This review will examine current concepts and challenges with existing PET imaging diagnostics for melanoma patients and introduce exciting new opportunities for PET in the current era of IO therapeutics.
Key points
- •
FDG PET/CT is highly sensitive and well suited for the non-invasive detection and monitoring of metastatic melanoma lesions.
- •
Tumor response assessment with conventional anatomic imaging approaches may be challenging in those melanoma patients treated with targeted or immune-oncology therapeutics.
- •
Recent advances in digital PET technology may enable new clinical approaches to assess whole-body tumor burden with higher image definition, faster image acquisition times, and at lower radiotracer doses.
Introduction
Melanoma remains the most deadly form of skin cancer, with an incidence that has risen faster than nearly any other cancer in the last 50 years. For stage I cutaneous malignant melanoma, the 5-year survival rate is 90%, whereas it is 15% to 20% for stage IV melanoma with distant metastatic disease. In the metastatic setting, it can spread to distant organs of the body through vascular and/or lymphatic spread. In addition, disease recurrence occurs in 50% to 80% of melanoma patients with locoregional metastatic involvement and almost all patients with distant metastases. An estimated 100,350 new cases of cutaneous melanoma were projected in 2020 with 6850 deaths. While the number of cases has been increasing, mortality rates are declining most likely due to promising new systemic therapies for the treatment of locally advanced and metastatic disease. In recent years, the development and clinical use of new IO therapeutics (ie, targeted small-molecule inhibitors and immunotherapy) have improved survival in melanoma patients. From 2013 until 2017, in men and women (ages 20–64 years), the overall mortality from melanoma dropped by 7% annually. During that same time for patients 65 years of age and older, mortality rates were declining by 5% to 6% per year, while prior to 2013, mortality rates were increasing. Imaging to assess for malignant/metastatic disease is a vital component of the work-up of patients with newly diagnosed lymph node-positive or recurrent melanoma so that the most appropriate therapy can be selected and delivered. Similarly, imaging plays a critical role in subsequently assessing the treatment response in patients with recurrent malignancy and/or metastatic disease.
Positron emission tomography with computed tomography (PET/CT) is clinically used for the detection and assessment of malignant/metastatic lesions in patients with melanoma as well as many other cancers. In the case of melanoma, 18 F-fluorodeoxyglucose (FDG) PET/CT imaging enables a whole-body assessment of physiologic and pathophysiologic glucose metabolism in order to identify metabolically reprogrammed cancer lesions, which demonstrate increased FDG uptake relative to the normal tissues nearby. FDG PET/CT can also provide insight into therapeutic responses of tumors to cytotoxic chemotherapy. Whereas conventional diagnostic imaging approaches with CT and magnetic resonance imaging (MRI) use anatomic changes in tumor size as the measure of treatment response, FDG PET/CT can provide additional functional insight by evaluating the metabolic activity of the tumor as another measure of treatment response. In particular, FDG PET/CT enables visual/qualitative assessment of glucose utilization throughout the body as well as semiquantitative measurement for evaluation of the metabolic response to therapy. The most widely used PET method for quantifying FDG activity is the standardized uptake value (SUV). In general, FDG PET/CT demonstrates high sensitivity and specificity for detecting and staging melanoma lesions when compared with CT and its improved accuracy can impact clinical and therapeutic management of melanoma patients.
The purpose of this review is to provide an overview of the current perspectives and future opportunities for PET imaging in the management of melanoma. Herein, we describe the current role of FDG PET/CT in the clinical management of melanoma including initial staging, treatment monitoring during therapy, restaging following therapy, and the subsequent detection of recurrent malignancy/metastatic disease. We also provide an overview of new developments in PET imaging technology including new and emerging imaging technologies, novel imaging approaches, and emerging concepts. Finally, we provide a brief perspective on clinical trials investigating the use of PET in melanoma patients to assess the response to therapies focusing primarily on immunotherapy.
Role of FDG PET in management of melanoma
Initial Staging
In order to determine the most optimal therapeutic plan for a newly diagnosed melanoma patient, the detection and localization of all sites of malignant/metastatic disease are essential. While the primary melanoma site and locoregional metastases may be detected on clinical examination, the detection of distant disease (including visceral metastases) is often more challenging and may not present until the disease is quite advanced and causing clinical symptoms. Furthermore, melanoma spreads distantly in an often atypical pattern with a high frequency of metastatic spread to the spleen, adrenal glands, and small bowel when compared to other malignancies. FDG PET/CT adds value as part of the comprehensive diagnostic evaluation of patients with advanced-stage or high-risk melanoma. Early studies evaluating the ability of FDG PET to detect distant metastatic disease are limited by the inclusion of patients with all stages of melanoma (ie, stages I–IV). In general, these studies showed FDG PET sensitivity ranging from 84% to 94%, and specificity ranging from 83% to 97%, compared to CT sensitivity of 55% to 58% and specificity of 70% to 84%. , Rodriguez Rivera and colleagues performed a meta-analysis on the use of FDG PET in patients with only stage III cutaneous melanoma. In this meta-analysis, the overall sensitivity and specificity of FDG PET in detecting metastatic disease were 89% and 89%, respectively, with a change in stage and/or management noted in 22% of patients. A systematic review from Krug and colleagues evaluating the utility of FDG PET for initial staging of cutaneous malignant melanoma included 2905 patients and patients with both early-stage and advanced disease. The pooled sensitivity and specificity for detection of metastasis by FDG PET was 83% and 85%, respectively, with disease management changes in 33% of patients. FDG PET was determined to be most helpful in patients with stages III and IV disease and especially in the detection of deep soft tissue, lymph node, and visceral metastases. Similarly, the diagnostic accuracy of FDG PET/CT was even higher for stages III and IV malignant melanoma when compared to stages I and II.
While the majority of evidence is retrospective with multiple systematic reviews and meta-analyses, there is limited prospective evidence highlighting the utility of FDG PET in the initial staging of patients with cutaneous melanoma. In fact, a prospective nonrandomized clinical trial of 144 patients with early-stage cutaneous melanoma showed no benefit with the addition of FDG PET to standard clinical work-up. Bastiaannet and colleagues performed a prospective comparison of FDG PET alone to CT in melanoma patients with palpable lymph node metastases. While FDG PET and CT tended to upstage patients by identifying the presence of distant metastases at similar rates in the study, FDG PET was able to identify more metastatic sites including the presence of bone and subcutaneous metastases when compared to CT alone. Hybrid FDG PET/CT imaging was then demonstrated to have a higher sensitivity than either FDG PET alone or CT in a separate meta-analysis evaluating multiple tumor histologies including melanoma.
Another prospective multicenter registry study was performed in Ontario evaluating the clinical utility of FDG PET/CT in patients with potentially resectable localized high-risk melanoma or recurrent disease being considered for metastasectomy. Of 319 patients included in this study, 18% were upstaged to M1 status following FDG PET/CT, which had a subsequent impact on surgical management of these patients. Another study demonstrated the clinical impact on patient management when using FDG PET/CT for melanoma patients being evaluated for metastectomy. In this study, half of the patients were subsequently spared surgery due to the detection of additional unresectable metastases and about 25% of patients had no change in the intended management plan. Therefore, FDG PET/CT can play an important role in initial surgical staging for those melanoma patients evaluated for potential metastectomy. For the detection of intracranial metastatic melanoma lesions, FDG PET performs poorly when compared with contrast-enhanced MRI and CT , although larger metastatic lesions may be detectable on FDG PET.
Sentinel lymph node biopsy is standard of care for initial staging of melanoma patients. It has been reported that the false-negative rate of sentinel lymph node biopsy is 6% to 29% and therefore new imaging approaches may be helpful in further detecting and quantifying metastatic nodal involvement. The status of the sentinel lymph node is the single most important predictor of survival in node-negative melanoma. To this end, FDG PET has a sensitivity of 17%, a positive predictive value of 50%, and a negative predictive value of 82%. A systematic review of pooled data from eight studies showed that FDG PET, in comparison to sentinel lymph node biopsy (SLNB), has a positive likelihood ratio (LR) of 1.33, a negative LR of 1.00, and a diagnostic odds ratio of 1.2. The Cochrane Collaboration has analyzed four studies evaluating PET/CT prior to SLNB, and found that PET/CT has a sensitivity of 10% and a specificity of 97%, which is inferior to a combination of ultrasound with fine needle aspirate of lymph nodes of concern prior to SLNB. It should be noted that these earlier studies likely utilized analogue photomultiplier tube-based PET detector imaging systems and these reported poor performance characteristics for PET detection of metastatic lymph nodes should not be surprising. Immunohistochemical evaluation of the excised sentinel lymph node(s) is capable of detecting isolated metastatic tumor cells, which would be below the detection limit of conventional analogue PET (cPET) and cPET/CT systems. As such, FDG cPET and FDG cPET/CT systems did not improve the detection of metastatic lymph nodes when compared with sentinel lymph node biopsy. More recently, the use of combined modality imaging with integrated FDG PET/MRI with diffusion weighted imaging also did not reliably differentiate metastatic lymph nodes from benign lymph nodes when correlated with sentinel lymph node biopsy. It is proposed that very small metabolic tumor volumes within metastatic lymph nodes may account for this historical poor sensitivity of FDG PET and therefore higher definition PET imaging approaches with smaller voxel volumes may improve the detection of subcentimeter metastatic deposits.
Given the limited prospective clinical evidence, imaging guidelines from the National Comprehensive Cancer Network (NCCN) suggest that cross-sectional imaging, including FDG PET/CT, should be considered in those melanoma patients with stage III disease for baseline staging and in patients with stage IV or recurrent disease.
Treatment Monitoring During Therapy and Restaging Following Completion of Therapy
As metastatic melanoma patients have poor prognoses, diagnostic imaging again serves as the noninvasive approach for evaluating treatment response to various oncologic therapies. In particular, FDG PET/CT readily assists in routine detection and response assessment of distant extracranial melanoma metastasis. , In the current era of immunotherapy/immune checkpoint inhibitor-based immuno-oncology (IO) treatments for melanoma patients, the imaging assessment of response to IO treatment has become more complex. These IO therapeutics have introduced new challenges for the interpretation of therapeutic response when compared with historical conventional cytotoxic chemotherapeutics. For example, small molecular IO inhibitors may improve patient survival while demonstrating minimal anatomic tumor size changes on follow-up diagnostic imaging. Due to the unconventional or delayed anatomic tumor responses of IO therapies on CT and/or MRI imaging, this challenge highlights the importance of adapting or developing new immune-related imaging response criteria strategies in patients treated with IO as opposed to cytotoxic chemotherapy. In the IO treatment setting, pseudo-progression is a phenomenon that presents as an initial enlargement of tumor size followed by a subsequent reduction in size. These initially enlarging tumors may result from immune-mediated tumor infiltrates and consequently these tumor infiltrates can increase FDG uptake on early PET imaging. It is important to recognize these early potential tumor pseudo-progression events in melanoma patients on IO therapies and to help to distinguish it from actual tumor disease progression on follow-up imaging. Some other imaging findings that can suggest therapy-related inflammatory response are reactive uptake in the lymph node drainage basins for malignant/metastatic lesions as well as diffusely increased FDG uptake in the spleen. These observations have resulted in the development of the immune-related Response Evaluation Criteria in Solid Tumor (irRECIST).
It is important to note that traditional methods to evaluate treatment response have focused on WHO, RECIST, and EORTC criteria, which were developed for cytotoxic therapy regimens as opposed to IO regimens. Per RECIST 1.1 criteria, PET/CT studies cannot independently be used for treatment response assessment because the attenuation-correction CT component of the PET/CT image acquisition is often deemed of inferior diagnostic quality when compared to dedicated diagnostic CT imaging owing to the lower radiation dose and lack of intravenous contrast administration on the attenuation-correction CT imaging for PET. Consequently, the majority of the seminal, prospective, therapeutic trials evaluating systemic therapies for stages III and IV melanoma (such as the Checkmate and KEYNOTE series) did not evaluate the role of PET in assessing clinical outcomes. Multiple clinical trials are now underway to address the role of PET in melanoma detection and response assessment. Table 1 highlights the current clinical trials that are assessing PET imaging at various time points and with various PET radiopharmaceuticals in melanoma patients treated with different IO therapeutics. Table 2 highlights the current international clinical trials incorporating PET imaging into the response assessment during IO therapy or the surveillance period following IO therapy for melanoma.
| Study ID | Radiopharmaceutical(s) | IO Therapy | Primary Endpoint | Imaging Time Points |
|---|---|---|---|---|
| NCT03356470 | FDG and FLT | Nivolumab or Pembrolizumab | Correlate baseline and posttreatment molecular imaging biomarkers of response to immunotherapy | Baseline and 10–12 wk posttherapy |
| NCT03089606 | FDG and [ 11 C]AMT | Pembrolizumab | Association of SUV max with objective response rate | Baseline and 12 wk posttherapy |
| NCT03888950 | FDG | Nivolumab or Pembrolizumab | Quantify changes in FDG uptake by PERCIST criteria | Baseline, days 21–31, and 3 mo posttherapy |
| NCT04272658 | FDG | Nivolumab or Pembrolizumab or combo Ipilimumab/Nivolumab | Differentiate progression vs pseudoprogression using 4D body-to-whole dynamic acquisition | Not specified |
| NCT03584334 | FDG | Nivolumab or Pembrolizumab | Threshold of FDG retention index to distinguish progression vs pseudo-progression | Baseline, 7 wk, and 3 mo posttherapy |
| NCT02716077 | FDG | Pembrolizumab | Disease-free survival | Not specified |
| NCT04221438 | FLT | Encorafenib and Binimetinib | Change in SUV max | Baseline and 8–9 wk posttherapy |
| NCT04462406 | FDG | Nivolumab or Pembrolizumab or combo with Ipilimumab | Event-free survival – active surveillance following negative PET or positive PET but negative biopsy | Baseline and 12 mo posttherapy |
| NCT03520634 | [ 18 F]PD-L1 | Nivolumab | Determine optimal dose of tracer and timing of imaging | Baseline and 6 wk posttherapy |
| NCT02591654 | FLT | Pembrolizumab | Prevalence of lesion detection | Baseline and 6 wk posttherapy |
| Study ID | Brief Description | Country Enrolling Patients |
|---|---|---|
| NCT03356470 | Comparing FDG PET and FLT PET, along with blood and tissue biomarkers | United States |
| NCT03888950 | Evaluate whether FDG PET predicts therapeutic response after two cycles of PD-1 directed therapy | France |
| NCT04272658 | Determine the value of 4D body-to-whole dynamic acquisition in FDG PET for immunotherapy monitoring | France |
| NCT03584334 | Using FDG PET to distinguish tumor progression vs pseudo-progression in patients with melanoma or non-small cell lung cancer | France |
| NCT02716077 | Early evaluation of response to pembrolizumab in patients with melanoma | United States |
| NCT04478318 | Determine the minimum FDG PET scan duration on a total-body vs conventional scanner | United States |
| NCT04462406 | Determine how FDG PET may allow early discontinuation of PD-1 directed therapy in unresectable stages IIIB–IV melanoma | United States |
| NCT03116412 | Prospective randomized multicenter trial to assess the role of imaging during follow-up after resection of stages IIb-c and III melanoma | Sweden |
| NCT03554083 | Determine the role of FDG PET in assessing response to neoadjuvant combination targeted and immunotherapy for patients with high-risk stage III melanoma | United States |
| NCT02621021 | Determine the role of FDG PET in assessing response to talimogene laherparepvec with or without radiation for patients with advanced melanoma, Merkel cell carcinoma, or other solid tumors with skin metastasis | United States |
| NCT02575404 | Determine the role of FDG PET in assessing response to GR-MD-02 plus pembrolizumab for patients with advanced melanoma, non-small cell lung cancer, or head and neck squamous cell cancer | United States |
| NCT04165967 | Determine the role of FDG PET in assessing response to adoptive tumor-infiltrating lymphocyte transfer plus nivolumab for patients with metastatic melanoma that failed immunotherapy | Switzerland |
| NCT03311308 | Correlate hypoxia measurements in tumor via FDG PET in advanced melanoma patients treated with pembrolizumab with or without metformin | United States |
| NCT03161756 | Determine the role of FDG PET in assessing response to denosumab plus nivolumab with or without ipilimumab for patients with metastatic melanoma | Australia |
| NCT04207086 | Determine the role of FDG PET in assessing response to neoadjuvant pembrolizumab plus levatinib for patients with resectable stages III/IV melanoma | Australia |
| NCT03475134 | Determine the role of FDG PET and 68 Ga-NODAGA-RGD PET in assessing response to tumor-infiltrating lymphocyte therapy plus nivolumab rescue for patients with unresectable locally advanced or metastatic melanoma | Switzerland |
| NCT02858921 | Determine the role of FDG PET in assessing response to neoadjuvant dabrafenib, trametinib, and/or pembrolizumab for patients with BRAF mutant resectable stage III melanoma | Australia |
It has been proposed that FDG PET may be able to detect and assess early metabolic responses in melanoma lesions to IO therapies as well as quantify changes in the whole-body metabolic tumor burden. On the other hand, persistently stable or increasing FDG avidity in tumor lesions treated with IO therapeutics may be an indicator of tumor resistance. It is likely that current and future clinical trials will also need to identify, characterize, and distinguish response patterns on FDG PET/CT for tumor response as well as nontarget tissue/organ toxicities that may develop during IO therapy. Such toxicities include dermatitis, inflammatory endocrinopathies, inflammatory esophageal/gastrointestinal manifestations, pneumonitis, hepatitis, etc. Many of these potential toxicities may be easily detectable on FDG PET/CT imaging in clinically asymptomatic patients undergoing routine imaging assessment.
More specifically, PET/CT may play a more important role in determining the functional/metabolic impacts of these IO therapies, especially when correlated with conventional anatomic imaging findings with CT and MRI. A systematic review and meta-analysis by Ayati and colleagues highlights the utility of various baseline PET parameters (ie, SUV peak , metabolic tumor volume, and total lesion glycolysis) as predictors of the final response to IO in patients with metastatic melanoma. Furthermore, PET-based response assessments using these various PET parameters improved sensitivity and specificity when compared to conventional imaging-based response criteria. PERCIST, PERCIMT, PECRIT, and EORTC 1999 criteria are other imaging assessment tools that have emerged which integrate PET/CT but are less frequently incorporated into clinical trials than RECIST. These PET criteria integrate various PET-specific metrics to determine treatment response, including features of target versus nontarget lesions, and the maximum voxel value of standardized uptake value (SUV max ). ,
New PET response assessment concepts and strategies for patients treated with IO therapeutics have led to a renewed interest in early interval PET imaging to better predict clinical response. On the interim FDG PET/CT imaging during IO, the presence of stable-appearing anatomic disease and relatively increased FDG avidity (ie, pseudo-progression) can represent early tumor inflammatory response (ie, favorable outcome), which will eventually demonstrate imaging findings on subsequent scans that are more consistent with tumor treatment/regression. In fact, evolving and new response assessment strategies, which integrate both anatomic and functional/metabolic metrics, may be more predictive of early and/or eventual response to IO.
Cho and colleagues performed a prospective study in patients with advanced melanoma treated with IO by serially monitoring treatment response via FDG PET/CT at days 21 to 28 and again at 4 months after the initiation of IO therapy. These authors used a combination of anatomic and functional imaging data collected at the early time points to develop criteria predictive of response to IO with 100% sensitivity, 93% specificity, and 95% accuracy. A similar study was performed by Sachpekidis and colleagues who evaluated the utility of interim FDG PET/CT performed following two cycles (6 weeks) of IO with response assessment based on tumor metabolic activity rather than anatomic tumor dimensions. A subsequent study from the same group demonstrated a threshold of four new FDG-avid tumor lesions on subsequent posttreatment FDG PET/CT imaging was a reliable indicator of IO treatment failure. Furthermore, as these new FDG-avid tumor lesions demonstrated diameters exceeding 1 cm in size, the sensitivity and specificity for treatment failure approached 90%. Similarly, modifications to the traditional PERCIST response assessment for patients treated with IO (ie, immunotherapy-modified PERCIST or imPERCIST) have demonstrated that new lesions, even in the setting of partial metabolic response or stable metabolic response, are metastatic in 55% of cases. Therefore, the detection of any new lesion should be considered indeterminate as opposed to immediately progressive disease and closely monitored on follow-up imaging or biopsied as clinically indicated.
In 2017, the PET/CT Criteria for early prediction of Response to Immune checkpoint inhibitor Therapy (PECRIT) and PET Response Evaluation Criteria for Immunotherapy (PERCIMT) were developed and proposed. In 2017, the revised Response Evaluation Criteria in Solid Tumor (RECIST) criteria for the evaluation of immunotherapy response (iRECIST) were also proposed. Annovazzi and colleagues assessed the predictive value of FDG PET/CT performed 3 to 4 months after initiation of IO and compared various PET metrics and response criteria. This retrospective study cohort consisted of patients treated with IO using either ipilimumab or with PD-1 inhibitors. Interestingly, for patients treated with ipilimumab, the metabolic tumor volume combined with PERCIMT criteria was the most reliable predictor for best overall response at 6 months, while for patients treated with PD-1 inhibitors, multiple PET metrics were found to be reliable predictors of response. Other authors have suggested that even earlier imaging time points following initiation of IO may be potentially predictive of IO treatment response (eg, 2 weeks following initiation of anti-PD1 therapy for advanced melanoma). The ability to quickly and reliably assess IO treatment efficacy or treatment resistance would enable the clinical determination of when to stop an ineffective IO therapy and switch to another therapy, thus reducing the financial burden and risk of future immune-related adverse events (irAEs) in those patients with treatment-resistant disease. , , FDG PET/CT is again useful in detecting the presence and resolution of irAEs. A retrospective review of 147 patients treated with IO for advanced melanoma who underwent either contrast-enhanced CT scan or FDG PET/CT, irAEs were detected with imaging in 31% of patients. Follow-up imaging was also helpful in monitoring for the resolution of irAEs.
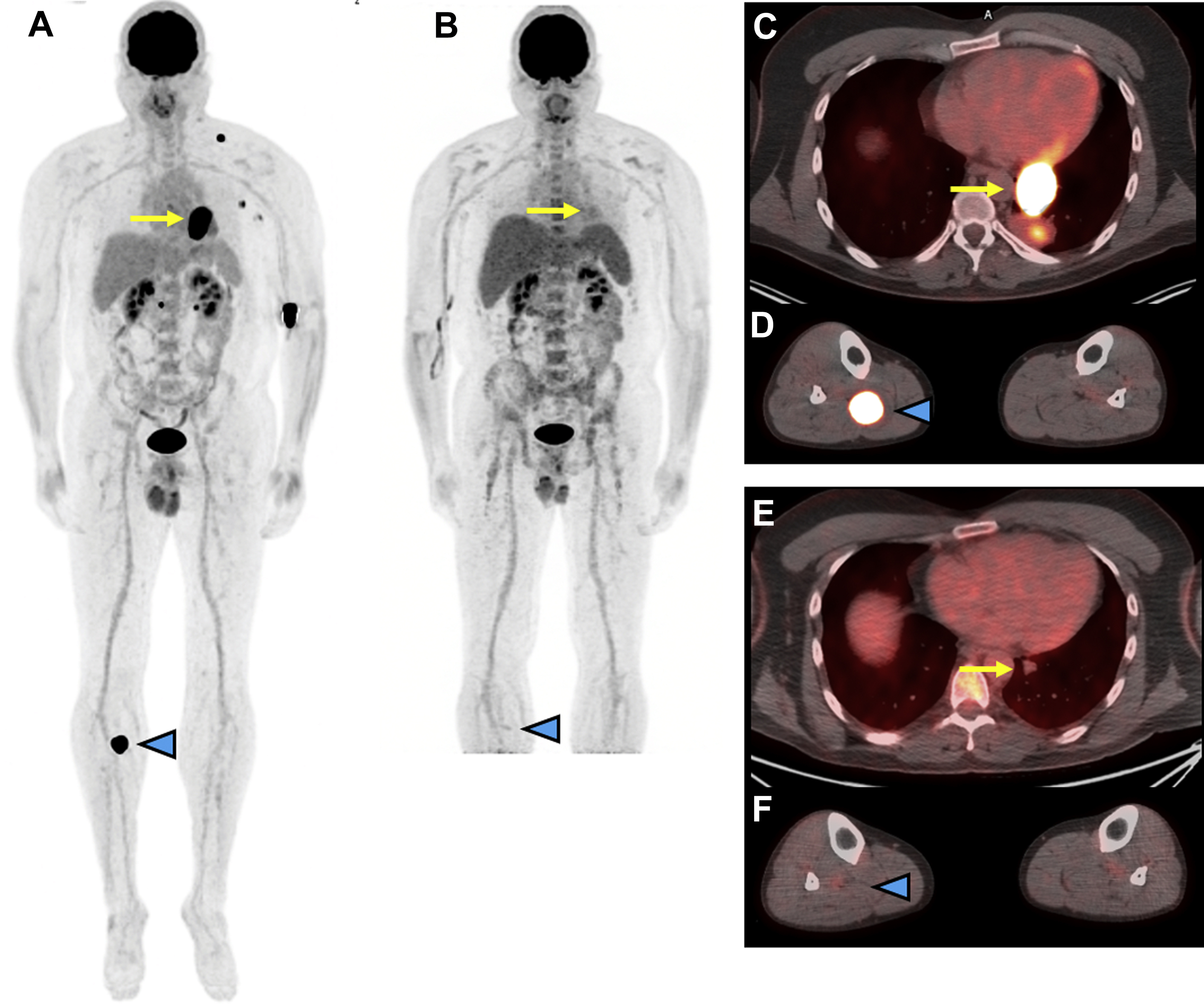
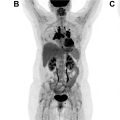
Within the NeoCombi trial, which evaluated the role of perioperative dabrafenib and trametinib therapy in resectable stages IIIB–C melanoma patients, 18 patients had evidence of a metabolic complete response on preoperative PET/CT. In addition, 11 of these 18 had both a complete response by RECIST criteria and a pathologic complete response in the resection specimen, whereas six patients with a metabolic complete response on PET/CT did not have a pathologic complete response in the resected specimen. In a different single institution phase Ib trial, a subset of six patients with resectable stages III/IV melanoma underwent pretreatment FDG PET/CT followed by just one dose of neoadjuvant pembrolizumab, follow-up FDG PET/CT 3 weeks later, and then surgical resection. In this subset of patients, a 20% decrease in tumor diameter using RECIST at 3 weeks following single-dose pembrolizumab was associated with treatment response in the surgical specimen but the change in FDG-avidity at 3 weeks following the single-dose IO administration was not yet predictive. While complete resolution of metastatic melanoma lesions on posttreatment imaging is the most comforting in terms of patient prognosis, data from a retrospective analysis of 104 patients treated with PD-1-directed IO for metastatic melanoma showed that CT imaging alone might be too conservative to predict treatment success. In this study, patients with a complete metabolic response on FDG PET/CT and a partial response on CT had comparable progression-free survival to patients with a complete response on CT. An example of using FDG PET/CT to monitor response to IO in metastatic melanoma is shown in Fig. 1 .