Abstract
Pregnancy is an inefficient process. A large proportion of early pregnancies end in miscarriage or early pregnancy failure. Ectopic pregnancies, those located outside of the proper uterine location, cause significant maternal morbidity and mortality. This chapter will review the utility of ultrasound in early pregnancy, when its use is essential in the differentiation of normal and abnormal gestation. The evaluation of pregnancy of unknown location, early pregnancy loss/failure, ectopic pregnancy, and cesarean scar pregnancy is reviewed. The ultrasound findings of normal and abnormal pregnancies are described. The synthesis of clinical, laboratory, and imaging findings is described. Emphasis is placed on accurate diagnosis without overdiagnosis, which can place normal pregnancy at risk.
Keywords
cesarean scar pregnancy, early pregnancy loss, early pregnancy failure, ectopic pregnancy, pregnancy of unknown location
Introduction
Pregnancy is an inefficient process. A large proportion of early pregnancies end in miscarriage or early pregnancy failure. Ectopic pregnancies (EP), those located outside of the proper uterine location, cause significant maternal morbidity and mortality. It is the role of the clinician to establish that the pregnancy is properly situated within the uterus and viable. This chapter will review the utility of ultrasound (US) in early pregnancy, when its use is essential in the differentiation of normal and abnormal gestation.
Pregnancy of Unknown Location
Definition
Pregnancy of unknown location (PUL) refers to a pregnancy whose location has yet to be properly identified and thus includes both pregnancies destined to be normal and those destined to be abnormal. The human chorionic gonadotropin (hCG) value is positive, but, the gestational sac has not been identified by US and there are no other US findings (e.g., echogenic free fluid; “bagel” sign) that would suggest an EP. This occurs early in gestation and represents a diagnostic dilemma for the clinician. Timely identification of a nonviable pregnancy allows prompt management, which may decrease complications and allow the woman to begin her processing of the loss. Timely identification of EP is crucial to minimize maternal morbidity and mortality and allows the broadest range of treatment options. However, overly aggressive treatment may lead to loss of a normal pregnancy.
Prevalence and Epidemiology
In reality, all pregnancies begin as PUL. Pain and bleeding are common during early pregnancy, and patients who present with these symptoms should be evaluated promptly to define location and viability of the pregnancy. Studies looking at the prevalence of PUL have reported that 5% to 42% of patients attending a specialized first-trimester clinic are given this diagnosis.
Etiology and Pathophysiology
Prior to 4 to 5 weeks from LMP, even high-frequency transvaginal US cannot discern the early pregnancy. Therefore, clinicians caring for women who present with pain and/or bleeding during this window are frequently faced with managing a PUL. Transvaginal US is essential in this scenario, and is guided by first-trimester developmental milestones and normal US findings, which are summarized in Table 44.1 .
| Gestational Age After LMP | Embryonic Milestone | Transvaginal Ultrasound Findings | Level of hCG (mIU/mL) |
|---|---|---|---|
| 23 days | Blastocyst implantation | Blastocyst is too small to visualize. Trilaminar normal appearance of endometrial lining | Variable |
| 3.5–4.5 weeks | Decidual changes at implantation site | Focal echogenic thickening | Variable |
| 4.5–5.5 weeks | Exocoelomic cavity of blastocyst | Gestational sac should be visible when it reaches 2–3 mm | 1000–2000 |
| 5–5.5 weeks | Yolk sac | Thin-walled cystic structure within gestational sac should be seen when gestational sac reaches 10 mm | 1000–7200 |
| 5–6 weeks | Embryo | Fetal pole is a focal echogenic area adjacent to yolk sac that should be seen when gestational sac is >10 mm | 7000–10,000 |
| 5–6 weeks | Cardiac activity | Should be seen when embryo is >5 mm |
These generalized “landmarks” cannot be precisely correlated to human chorionic gonadotropin (hCG) values because the normal ranges of hCG levels at different gestational ages are too wide. Serial USs, rather than absolute hCG values, can be used in evaluation when EP has been excluded. After an intrauterine pregnancy (IUP) has been confirmed, serial USs can be performed if there are doubts about pregnancy viability to avoid unnecessarily interrupting a wanted IUP, while still recognizing abnormal pregnancy as early as possible.
Gestational Sac: During the first 3 to 5 weeks of gestation, the endometrium has an echogenic appearance as the hormonal mileu causes a decidual reaction. This appearance is nondiagnostic because it can be seen in late secretory phase of a nonpregnant uterus as well ( Fig. 44.1A ). Gestational sac is an US, not an anatomic term, used to describe the sonolucent round structure eccentrically located in the endometrium that represents the fluid-filled chorionic cavity (see Fig. 44.1B ). The chorionic sac, the echogenic rim, is produced by the developing chorionic villi involving maternal decidual tissue and is termed a decidual reaction; it already contains the amnion, trilaminar embryonic disk, and yolk sac, but at this gestational age these are too small to be distinguished by US ( Fig. 44.2 ). Depending on the US equipment employed and on the observer, the gestational sac can be measured when it is 2 to 3 mm, but it is normally seen at 5 mm in most settings, typical of 5 weeks’ gestation. While the thick decidual sign and a small 2 to 3 mm sac embedded on one side of the echogenic cavity line in the endometrium correlates with an intrauterine pregnancy, its absence does not necessarily imply a failed or EP. Gestational age can be estimated by using the mean sac diameter (MSD), derived from averaging three orthogonal sac diameters, which can be correlated to gestational age. A simple way of estimating gestational age from the sac size is to add 30 to the MSD in millimeters to get gestational age in days: Menstrual age (days) = MSD (mm) + 30.


Once the embryo is detected, it should be used for dating. The gestational sac grows approximately 1 mm/day, so there is little utility in repeating US scans for viability check or dating purposes sooner than 4 to 7 days after the prior scan.
An actual IUP is not confirmed until a yolk sac is seen. A fluid collection (i.e., blood) in the endometrial cavity, which sometimes shifts back and forth moved by shallow uterine activity of a woman with EP, is called a pseudosac; it can be mistaken for an intrauterine gestational sac, especially if scanning transabdominally. Features such as location within the middle of the uterine cavity flanked by the anterior and posterior endometrial layers, elongated shape, and absence of gestational reaction all increase the level of suspicion. A presumed pseudosac, however, is not diagnostic of EP because of a high false-positive rate, and some practitioners have abandoned the term.
Yolk Sac : The yolk sac is a round structure with a sonolucent center and echogenic periphery, located within the gestational sac. With high-frequency US transducers it can usually be seen by the time the gestational sac reaches 5 mm and is the first definitive sign on an intrauterine gestation. Given individual variability, this sac size should not be used as a cutoff, and a follow-up US scan and correlation with hCG should be done. The yolk sac continues to grow up to 6 mm by 10 weeks’ gestation and then migrates to the periphery of the chorionic cavity, becoming undetectable by the end of the first trimester ( Fig. 44.3 ).
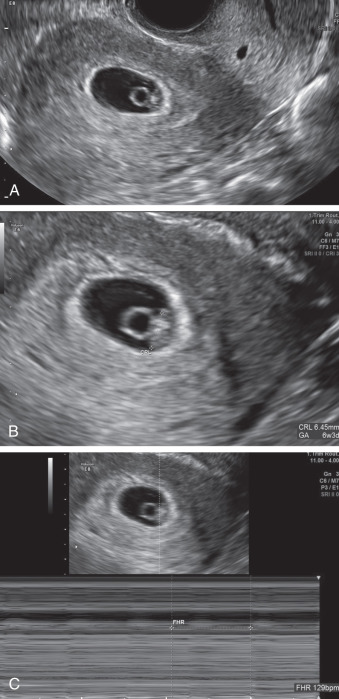
Crown-rump length (CRL): the embryonic disk (also referred to as a fetal pole, or more correctly, embryonic pole ) is a thickened region along the outermost part of the yolk sac (see Fig. 44.3 ). It becomes visible when it is 1 to 2 mm in length. An embryo is usually seen by the time MSD reaches 18 mm, and lack of embryo by an MSD of 25 mm is diagnostic of early pregnancy loss.
CRL is the longest linear measurement of the embryo, and the preferred dating measurement up to 14 weeks’ gestation. However, before 18 mm, a true “crown” and “rump” do not exist, and measurements are taken along the long axis of the embryo (the greatest measurement should be used). As the embryo grows, the rostral neuropore closes and develops into the forebrain, and the caudal neuropore elongates into a tail. The CRL generally increases 1 mm per day. This daily increase can be useful when evaluating a suspected failed pregnancy. Similar to MSD, modern US machines contain formulas to calculate gestational age from CRL.
Cardiac activity: several older studies of normal pregnancies suggested that cardiac activity should be routinely seen by an embryonic size of 4 mm. However, more recent literature recommends to use embryonic size of 7 mm. The heart rate can be calculated via M-mode or spectral Doppler; color/power Doppler should be used for the minimal time possible time or completely avoided due to its potential bioeffects, with a concentration of energy on the embryo. The primitive heart at this early stage is very prominent and can often be detected before the embryo is seen separate from the adjacent yolk sac (see Fig. 44.3C ).
Manifesations of Disease
Clinical Presentation
Patients with PUL present with amenorrhea and a positive pregnancy test. They may also have pain, bleeding, or cramping. Patients with risk factors for EP (previous ectopic, tubal pathology) should be monitored closely until pregnancy location has been established.
Physical exam is usually benign. Tenderness should prompt evaluation for EP as described following.
Imaging Technique and Findings
Ultrasound.
Transvaginal US was first used in 1987 to image an IUP. Since then, it has become the imaging modality of choice, and is considered superior to transabdominal US because it allows for earlier and more reliable detection of pregnancy location and fetal viability. Transabdominal US (performed before a patient voids) with transducer frequency of 3.5 to 5 MHz and greater is a good starting point to get an overview of the pelvis because of its larger field of view, which is helpful when looking for the signs of hemoperitoneum and evaluating the Morrison Pouch. With an empty bladder, a transvaginal transducer with frequency of 5 to 12 MHz can be positioned closer to the pelvic organs, resulting in improved resolution while still providing adequate penetration. Doppler can be used if indicated, although embryonic exposure to Doppler should be minimized to reduce the risks of bioeffects in any first-trimester US. Doppler is especially useful when examining the ovaries for the corpus luteum, which typically has a “ring of fire” appearance ( Fig. 44.4 ); however, a tubal ectopic may also demonstrate a ring of fire.

US in PUL lacks the diagnostic findings of intrauterine pregnancy listed above. In addition, no gestation is found outside of the normal uterine location. The ovaries may demonstrate a corpus luteum, which should not be confused with an ovarian EP, a rare occurrence. Free fluid may be noted in the pelvis. If it is echogenic, it may be a sign of hemorrhage from an EP or a corpus luteum, and must be examined further.
Magnetic Resonance Imaging.
MRI has a limited role in the evaluation of patients with PUL. In circumstances when rare types of EP such as abdominal or interstitial (ectopic) pregnancy are suspected, it may be useful as an adjunct to transvaginal sonography.
Classic signs of PUL are positive hCG and no sonographic evidence of an intrauterine or EP.
Synopsis of Treatment Options
Prenatal
As long as the patient is stable, management is expectant until definitive diagnosis may be made of intrauterine (viable or nonviable) or EP (see the following discussion on EPF and EP on how these diagnoses are made). Patients are followed with serial hCG measurements, usually 48 hours apart. The use of progesterone level(s) in the management of PUL has been proposed by many authors. In a recent publication using a two-step approach, a level of progesterone of less than or equal to 2 nmol/L was used for initial triage of patients. Those with progesterone levels greater than 2 had a serum hCG 48 hours later, and then were triaged according to the combined results. Patients with low progesterone were more likely to have failed early IUP. This protocol resulted in a more efficient classification of PUL as low-risk (those destined to have either a normal or failed IUP) and those at high risk for complications (EP).
US performed with critical threshold of hCG should demonstrate intrauterine findings as listed above. Failure to confirm an IUP should lead to treatment for EP, as discussed later in the section Ectopic Pregnancy.
In an unstable patient, exploratory laparoscopy with or without uterine sampling is suggested.
Failed Pregnancy—Early Pregnancy Loss or Failure (EPF)
Definition
Early pregnancy loss or early pregnancy failure refers to a nonviable pregnancy within the first 6 to 7 weeks of gestation. This term encompasses an empty gestational sac as well as an embryo with no cardiac activity. Missed abortion was used in the past in this context, but is now an archaic term.
Prevalence and Epidemiology
Estimates of the frequency of EPF vary depending on the patient population. Some studies look at women undergoing routine examinations, others look at patients with pregnancies from in vitro fertilization, and still others follow patients presenting to the emergency department with complaints. It also depends on whether or not loss is recognized and whether bleeding is present or not. Prevalence has a wide range; EPF has been reported in 8% to 31% of all pregnancies.
Etiology and Pathophysiology
Vaginal bleeding in the first trimester occurs in about 25% of all pregnancies; about half of first-trimester pregnant women who bleed ultimately miscarry. Multiple causes of EPF exist. The most common etiology is a chromosomal abnormality; the most frequent chromosomal abnormality is autosomal trisomy, but polyploidy, monosomy, unbalanced translocations, and structural rearrangements have been described. A long list of other etiologies, including endocrine factors (uncontrolled diabetes mellitus and hypothyroidism), uterine factors (congenital uterine anomalies, fibroids, intrauterine adhesions), immunologic factors (antiphospholipid syndrome, systemic lupus erythematosus), advanced maternal age, and environmental factors (smoking, drug use, teratogens), can also contribute. In most cases, no work-up is indicated, and most future pregnancies are successful. In some cases, the etiology remains unexplained. It is important to ensure that patients do not blame themselves or their level of physical activity for the miscarriage.
Manifesations of Disease
Clinical Presentation
Women with EPF present with amenorrhea and a positive pregnancy test. Vaginal bleeding and pelvic pain are also present if a patient is in the process of having a spontaneous abortion. On examination, the cervix might be closed or open, and the products of conception (POC) may be seen in the vagina or the cervical orifice (os), if they have not already been passed. The finding of early pregnancy loss may also be incidental on US if the patient is asymptomatic. Table 44.2 summarizes clinical presentation in EPF.
| Type | Clinical Findings | Ultrasound Findings |
|---|---|---|
| Threatened abortion | Vaginal bleeding and cramping | Normal-appearing IUP |
| Cervix is closed | ||
| Early pregnancy loss/failure | Vaginal bleeding and cramping may or may not be present | IUP without a heartbeat |
| Cervix is closed | Anembryonic pregnancy | |
| No expulsion of products has occurred | ||
| Inevitable abortion | Bleeding and pain worsens | Viable or nonviable IUP |
| Cervix is dilated | ||
| Products might be seen through the internal os, but no expulsion has occurred | ||
| Incomplete abortion | Vaginal bleeding and cramping, partial passage of POC | Blood and trophoblastic tissue, endometrial thickness of any size, heterogeneous tissue with or without gestational sac; this tissue is referred to as POC and distinguishes complete from incomplete abortion |
| Cervix is open or closed | ||
| Retained POC | Vaginal bleeding and cramping | Placental, fetal, or embryonic tissue or trophoblast that remains in the uterus after spontaneous pregnancy loss, termination, or delivery |
| Cervix is open or closed | ||
| Complete abortion | Vaginal bleeding and cramping, complete passage of POC | Empty uterus with normal appearance of endometrium |
| Cervix is open or closed | ||
| Septic abortion | Abdominal pain, fever, purulent discharge, symptoms of sepsis | POC |
| Cervix is open or closed |
Imaging Technique and Findings
Ultrasound.
As with PUL, transvaginal US is the imaging modality of choice. An initial transabdominal US may be useful as a road map as discussed previously.
Early pregnancy loss may present on US as demise of a previously live embryo, an embryo in which cardiac activity has never been noted, or an anembryonic gestation, in which an embryo has never been identified.
Fig. 44.5 shows an anembryonic intrauterine gestation, previously termed a blighted ovum . A large empty sac might be a result of embryonic demise and resorption that has occurred before presentation. The gestational sac might also appear as flat and irregular in shape.