CHAPTER 24
Prenatal Intervention in the Fetus with Cardiac Disease
Modern-day treatment of congenital heart disease has been redefined by the success of neonatal surgery to address complex structural disorders. Conditions that were palliated or lethal are now routinely addressed within the first days of life. Tetralogy of Fallot, historically not repaired until early childhood, is routinely corrected by 3 to 4 months of age to promote normal development of the right ventricle and pulmonary arteries.1–4 In addition, for complex cardiac lesions that remain unrepairable, such as hypoplastic left heart syndrome, successful palliative operations have been developed with remarkably good outcomes. Many centers now report survival rates for the Norwood palliative approach for hypoplastic left heart syndrome as high as 80% to 90% at 5 years of life.5,6
Fetal intervention for cardiac lesions is not a new concept. It was tried with limited technical success 15 to 20 years ago, but with no evidence of overall improvement in cardiac performance, it was not aggressively pursued.7–9 The advent of improved ultrasound equipment and interventional technology led to renewed interest in prenatal intervention. The concept was aggressively revisited just after the turn of the millennium, with published reports of technical success and safety in 2004.10,11
Candidate Lesions
Critical Aortic Stenosis
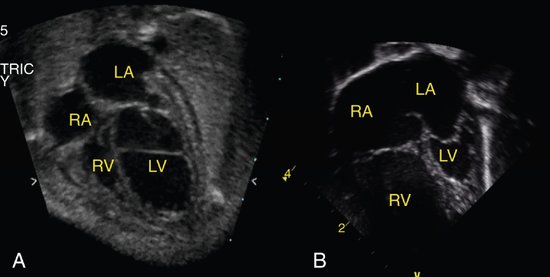
Critical aortic stenosis has, perhaps, been the lesion generating the most interest in fetal intervention. Although mild disease may be well tolerated in a fetus and only require postnatal balloon valvuloplasty, severe obstruction typically presents in the second trimester with left ventricular enlargement and dysfunction. Studies have shown the natural history of this lesion is to develop progressive dysfunction and endocardial fibroelastosis with disproportionate growth, resulting in functional hypoplastic left heart syndrome (Fig. 24–1).12 In hypoplastic left heart syndrome, the left heart is severely underdeveloped and dysfunctional and therefore cannot support a normal stroke volume and cardiac output. The postnatal palliative approach to address this inadequacy is the Norwood palliation, which subjects the child to a series of surgeries designed to utilize the single functional right ventricle to support both the pulmonary and systemic circulations. Despite the remarkable advances in surgical techniques and perioperative care with improved early survival, the long-term prognosis for these patients remains guarded at best.
Sonographically, several features can be evaluated to help determine the severity of the aortic valve disease and therefore the risk of progression to hypoplastic left heart syndrome. Typically, the aortic valve is small with thickened, severely immobile leaflets (Fig. 24–2) and only a tiny jet of antegrade flow into the ascending aorta by color or pulsed Doppler imaging. The papillary muscles and endocardium of the left ventricle are often echogenic, suggesting the presence of scar tissue or endocardial fibroelastosis. Flow across the foramen ovale is reversed as a result of left ventricular dysfunction and resultant high filling pressure in the left atrium. Finally, as the cardiac output decreases because of valve obstruction and left ventricular dysfunction, the flow through the aortic arch reverses, with the right ventricular output coursing across the ductus arteriosus and retrograde through the arch to perfuse the upper body in the absence of adequate forward flow. The Doppler gradient across the aortic valve is not typically useful to estimate the severity of disease because this may only be a sign of poor ventricular function. In rare cases the left heart can enlarge so severely that it compromises the right ventricular cardiac performance, causing fetal hydrops and death.
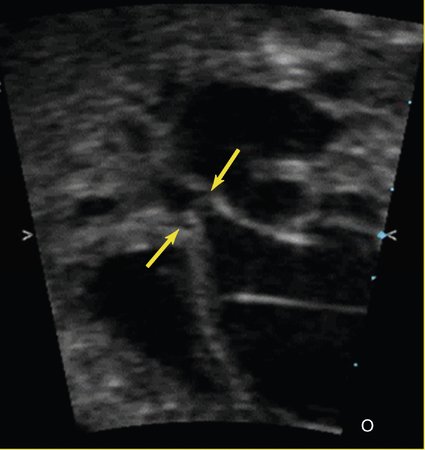
Hypoplastic Left Heart Syndrome with Intact Atrial Septum
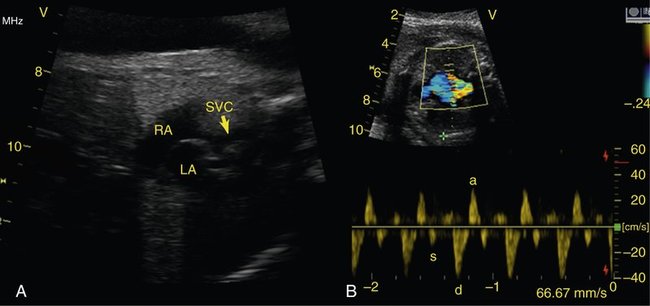
Another lesion potentially amenable to fetal intervention is the atrial septum when it is restrictive or intact in the setting of hypoplastic left heart syndrome. Although, as previously mentioned, the survival rates for hypoplastic left heart syndrome are considerably higher than they used to be, in 5% to 10% of cases the foramen ovale can become severely restrictive or close completely. After delivery this results in an inability to adequately drain the pulmonary vascular bed as a result of distal obstruction and the inability to decompress across the foramen ovale (Fig. 24–3). Historically, the prognosis for these infants has been extremely poor, with mortality rates as high at 90% with death typically occurring immediately after birth. Despite new aggressive therapy, including delivery in the operating room or catheterization laboratory for immediate intervention, the mortality rate remains at 60% to 70% at best. One explanation for these continued poor outcomes may be the period of time in utero during which the pulmonary venous system cannot adequately drain, causing damage to the pulmonary venous system. Examination of the postmortem lungs reveals arterialization of the pulmonary veins, which likely contributes to the persistent pulmonary edema and high vascular resistance that occurs despite adequate decompression of the left atrium through a newly created atrial septal communication.13
Pulmonary Atresia with Intact Ventricular Septum
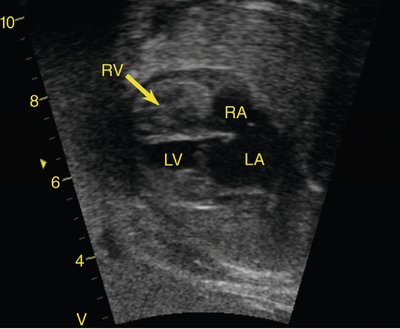
Similar to the left side, when there is severe obstruction of the right heart without a communication between the two ventricles, the right ventricle and tricuspid valve become hypoplastic (Fig. 24–4). In patients where the tricuspid valve appears of adequate size, postnatal ballooning of the pulmonary valve can lead to remodeling and growth of the right ventricle with a functionally normal right side. However, when the tricuspid valve or the right ventricle are severely underdeveloped to the point where they cannot sustain a normal cardiac output, the result is hypoplastic right heart syndrome with reliance on the left ventricle as the single circulating pump. The outcome for this disease is similar to that of hypoplastic left heart syndrome. Although the single left ventricle is better able to handle the workload than is a single right ventricle, the significant incidence of right ventricle–to–coronary artery fistulas and coronary artery stenosis results in relatively high mortality rates, with a 5-year survival rate of approximately 65%.14–16
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree