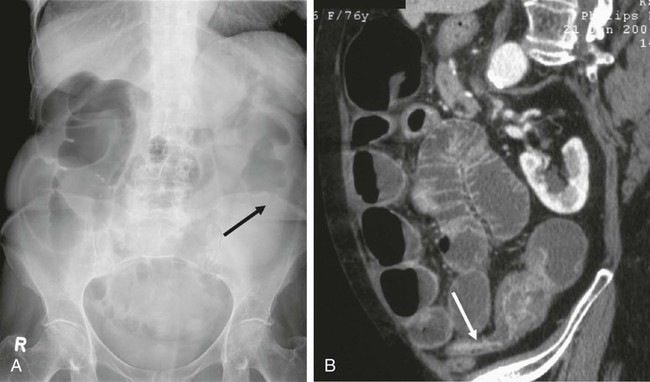
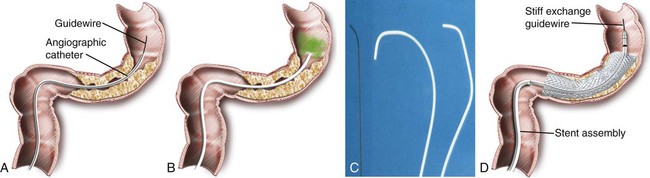
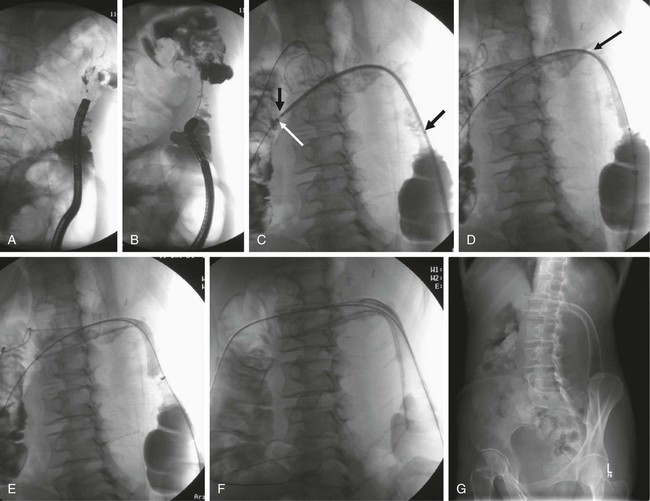
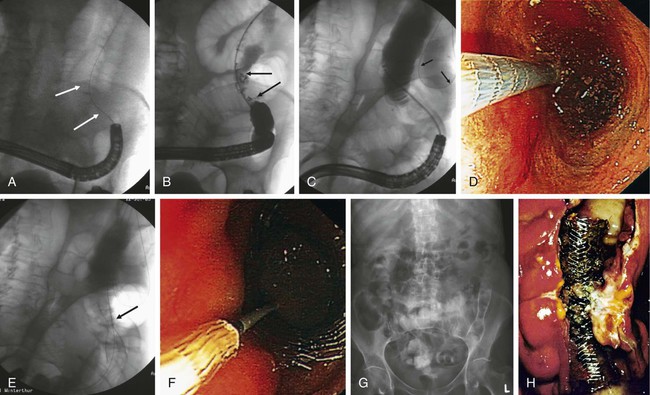
Chapter 131 Colorectal cancer is the fourth leading cause of cancer death in the United States after lung cancer and carcinoma of the breast and prostate.1 Close to a million new cases of colorectal cancer are detected each year worldwide, and almost 500,000 deaths are attributed to malignant tumors of the colon and rectum.2 Up to 30% of these patients experience acute partial or complete obstruction, with up to 75% of colorectal cancers occurring in the left colon.3 Other, less frequent causes of colorectal obstruction include malignant infiltration from adjacent malignant tumors or metastatic involvement. Benign obstruction such as diverticulitis or other inflammatory bowel diseases and anastomotic or postirradiation strictures are rare. Colonic cancer causing obstruction tends to be at a more advanced stage when first seen, and a higher proportion of obstructed patients (27%-40%) than unobstructed patients already have liver metastases.4 Curative surgery is not feasible in up to 30%. The advanced stage is also reflected in the poor 5-year survival rate of less than 20% with obstructive disease.4 Acute obstruction of the large bowel usually requires urgent surgical treatment, and these patients generally are high surgical risks because of poor general condition related to dehydration and electrolyte imbalance. Mortality and operative morbidity are in the range of 10% to 20% and 40% to 50%, repectively.5 If patients can be treated electively, mortality rates drop to 3.5% to 7% and operative morbidity to 2% to 24%.6,7 Therefore, for many years the standard treatment of acute malignant obstruction of the colon has been a two- or three-stage procedure (Hartmann procedure) consisting of first a decompressing colostomy, including resection of the primary tumor at the same time or in an additional operation, and finally closure of the colostomy.3 Both approaches result in long hospital stays, and up to 62% of these patients never undergo closure of the colostomy.4,8 The best oncologic approach to obstructing carcinoma of the colon is primary resection without colostomy. To facilitate primary anastomosis and avoid colostomy, several techniques such as nasointestinal suction and lavage,9 colonic decompression with rectal-colonic tubes,10 lavage during surgery,10 and tube cecostomy3 have been used to relieve acute distention of the colon and rectum. However, nasointestinal suction and lavage require several days to become effective, rectal-colonic tubes may be impossible to place in patients with severe tumor stenosis, and tube cecostomy is unreliable because of tube obstruction.3 Nonsurgical methods such as balloon dilatation11 and laser recanalization12 have been used occasionally. Therefore, self-expanding metal stents (SEMS) have been advocated to serve as a bridge to surgery for rapid relief of obstruction and allow the patient to be prepared for elective tumor resection with primary anastomosis. Likewise, stents are used as a method of definitive palliation for colonic obstruction in patients who are not surgical candidates, thereby obviating the need for colostomy.5 The primary indications for placement of endoluminal colorectal stents are for (1) bridge to surgery in patients with resectable colonic obstruction to allow efficient bowel cleansing and single-stage surgical resection and (2) long-term palliative colonic decompression to avoid colostomy in patients with unresectable obstruction.5,13–15 Other, less common indications are benign conditions such as preoperative stenting in diverticular disease.16,17 Rarely, anastomotic, ischemic, and radiation-induced strictures are treated with stents, and finally, colonic fistulas may be handled with covered stents.18–23 Clinical or radiologic evidence of intestinal perforation and distal rectal lesions where a safe landing zone of at least 2 cm above the anal sphincter cannot be obtained are absolute contraindications to stent placement. Colorectal obstruction that is too long or patients with multilevel obstruction should not be stented. Tumors that cannot be passed by a guidewire and stent cannot be treated.13,14 In the majority of cases, colonic stenting is performed in an emergency setting requiring an appropriately trained team of interventional radiologists, endoscopists, nursing staff for patient monitoring (including administration of neuroleptanalgesia), and availability of high-quality fluoroscopy.5 Standard angiographic catheters such as the Cobra or Headhunter type are used in combination with steerable and hydrophilic guidewires (Terumo, Tokyo, Japan) to negotiate the obstruction. Stiff/superstiff guidewires (Amplatz Super Stiff wire, Lunderquist wire [Boston Scientific, Natick, Mass.]) are necessary to advance the stent-bearing instruments. Endoscopic guidance is used at institutions where the procedure is performed by gastroenterologists. A variety of SEMS specifically designed for colonic use is available today (Table 131-1). They are manufactured from stainless steel, Elgiloy (cobalt-chromium-nickel), or nitinol (nickel-titanium). Postdeployment diameters vary from 18 to 30 mm, and typical unconstraint lengths are 6 to 12 cm (see Table 131-1). Most modern stents are flared at one or even both ends to prevent migration, particularly in covered versions. For many SEMS, the delivery systems are provided for per anus use over the wire (OTW) as well as for through the scope (TTS). Colonic stents should be flexible enough to allow easy placement along acute curves and bends, particularly in the case of an elongated sigmoid colon or around the colonic flexures. The Enteral Unistep Colonic Wallstent (Boston Scientific), with a maximum diameter of 22 mm, was most frequently used13,24–28 until the early 2000s. Recently, more atraumatic and flexible nitinol modifications, such as the Ultraflex Precision Colonic Stent for OTW application29 and the WallFlex Colonic Stent for both OTW and TTS use (Boston Scientific), have been introduced (Fig. 131-1; see Table 131-1). Because of its atraumatic ends and proximal tulip shape (27/30 mm) to reduce the risk of perforation and migration, and the availability of both OTW and TTS systems, the WallFlex is our standard stent for colonic obstruction and is used at many other institutions.28,30,31 TABLE 131-1 Commercially Available Colonic Stents OTW, Over the wire; prox., proximal; PTFE, polytetrafluoroethylene; TTS, through the scope. The Colonic Z-Stent (Cook Endoscopy, Winston-Salem, N.C.) has a 31F introducer system of only 40-cm in length, limiting its application to distal lesions. Recently a 10F TTS nitinol version has been released (see Table 131-1). Other covered and uncovered stents, such as the HANAROSTENT (M.I.Tech, Seoul, Korea) and Niti-S Colorectal Stent (Taewoong Medical, Seoul, Korea), have either flanges on each side (dogbone) and/or uncovered bare ends, or have an extra outer self-expanding bare nitinol stent body (Niti-S ComVi [Taewoong Medical]) to prevent migration (see Table 131-1). However, apart from treating malignant fistulae, iatrogenic perforation, or postsurgery leaks, covered stents seem not to have a significant advantage and have not gained wider acceptance in clinical practice in the United States and Europe because of their unavailability.5,32,33 The SX-ELLA Colorectal-Enterella Stent (ELLA-CS, Hradec Králové, Czech Republic) is repositionable and retrievable, thanks to a plastic loop at the distal end, and may be particularly suited for benign or rectal lesions.26,34,35 Modern nitinol and Elgiloy SEMS are nonferromagnetic and therefore compatible with magnetic resonance imaging (MRI). A radiologic diagnosis of acute colonic obstruction is made by plain film radiography and computed tomography (CT) (Fig. 131-2). Barium enema, colonoscopy, and biopsy can help determine the exact location and nature of the obstruction. CT is also the method of choice for staging and may provide important information about the presence of multiple stenoses or concomitant small bowel obstruction in peritoneal carcinosis that have to be ruled out before placement of colonic stents. CT colonography may be of additional value. The stenosis can be negotiated (1) primarily with radiologic techniques alone, with optional endoscopy reserved for difficult cases,5,26,29,36,37 (2) with hybrid endoscopic-radiologic methods,13,17,25,26 and (3) endoscopic stent placement with limited fluoroscopy, probably the preferred method at many institutions where gastroenterologists are in the lead of gastrointestinal stenting.28,30 Lesions in the proximal portions of the colon are best handled with a combined endoscopic-fluoroscopic approach to overcome the tortuosity of the colonic flexures.13,17 Patients are best treated under mild sedation and placed in a left lateral position on the fluoroscopy table to start. A high-torque (7F) angiographic catheter (Headhunter or Cobra type) or a guiding catheter is placed in the colon, and the colonic segments are best negotiated with a catheter-guidewire combination (Fig. 131-3, A-C). Water-soluble contrast material is used to outline the bowel lumen and the area of the stricture. Conventional or hydrophilic guidewires, or both, may be used. Once the stricture has been passed with the catheter-guidewire assembly, the lesion is best defined by injecting contrast material through a catheter with multiple side holes and rotating the patient to an optimal position for stent insertion. For stent placement, an exchange-length superstiff wire (Amplatz Super Stiff, Lunderquist [Boston Scientific]) is placed well beyond the lesion. After advancing the stent to the desired location under fluoroscopic control, the stent is released (Fig. 131-3, D). If acute bends or kinks have to be passed or an elongated rectosigmoidal arch reduces forward pushability, a stiff large-bore guiding catheter (i.e., 11F Mullin sheath [William Cook Europe, Bjaeverskov, Denmark]) may be helpful to advance the stent assembly to the desired location. Patients are mildly sedated with midazolam and given pain medication (pethidine chlorhydrate). With the patient in the supine or oblique decubitus position on the fluoroscopy table, the endoscope is advanced to the distal end of the stenosis, and the obstruction is passed with a guidewire under endoscopic and fluoroscopic guidance (Fig. 131-4, A). Various types of steerable and hydrophilic guidewires such as the Glidewire (Terumo Medical Corp., Somerset, N.J.), Zebra (Boston Scientific), or endoscopic wires (Wilson-Cook Medical, Winston-Salem, N.C.) may be used to pass the obstruction. After placement of a multi-sidehole endoscopic catheter with its tip passed beyond the obstruction, the extent of the stenosis is visualized with the injection of water-soluble contrast material (Telebrix, Guerbet, France) (Fig. 131-4, B). For stent placement, a stiff guidewire at least 400 cm in length (Zebra), with its tip well beyond the obstruction, has to be used to accept any stent delivery system that will fit through the working channel of the endoscope (Fig. 131-4, C). The stent is deployed under combined fluoroscopic and endoscopic control such that the middle of the stent covers the stricture, with the stent extending at least 1 to 2 cm on each end of the lesion (Fig. 131-4, D-F). If a lesion is not adequately covered, an additional stent must be placed. A plain film is taken 24 to 48 hours after stent placement to confirm adequate position and expansion of the stent, as well as regression of the ileus, and to exclude inadvertant perforation (Fig. 131-4, G). Figure 131-4, H, shows a resected specimen with the stent in place. As a general rule, intraluminal position of guidewires and catheters must be confirmed at all times. The significant shortening of braided stents during release must always be taken into account; stents must have sufficient length to adapt to curves and bends and prevent protrusion of bare stent ends (particularly those of the open-end type, such as the Enteral Wallstent) or obstruction by abutting of the free stent end against the bowel wall. Therefore, placement of the free edges of the stent near sites of bowel angulation should be avoided and treated with additional stents if necessary (Fig. 131-5). Stents placed too close to the anal sphincter may cause tenesmus and incontinence5,18 and carry a higher risk for expulsion. Balloon dilatation before stent placement should be avoided because of the increased risk for perforation or potential metastatic spread.5,16,17,28,29 In our experience, additional balloon dilatation to adequately expand the stent is only rarely necessary (see Fig. 131-5, D and E
Preoperative and Palliative Colonic Stenting
Indications
Contraindications
Equipment
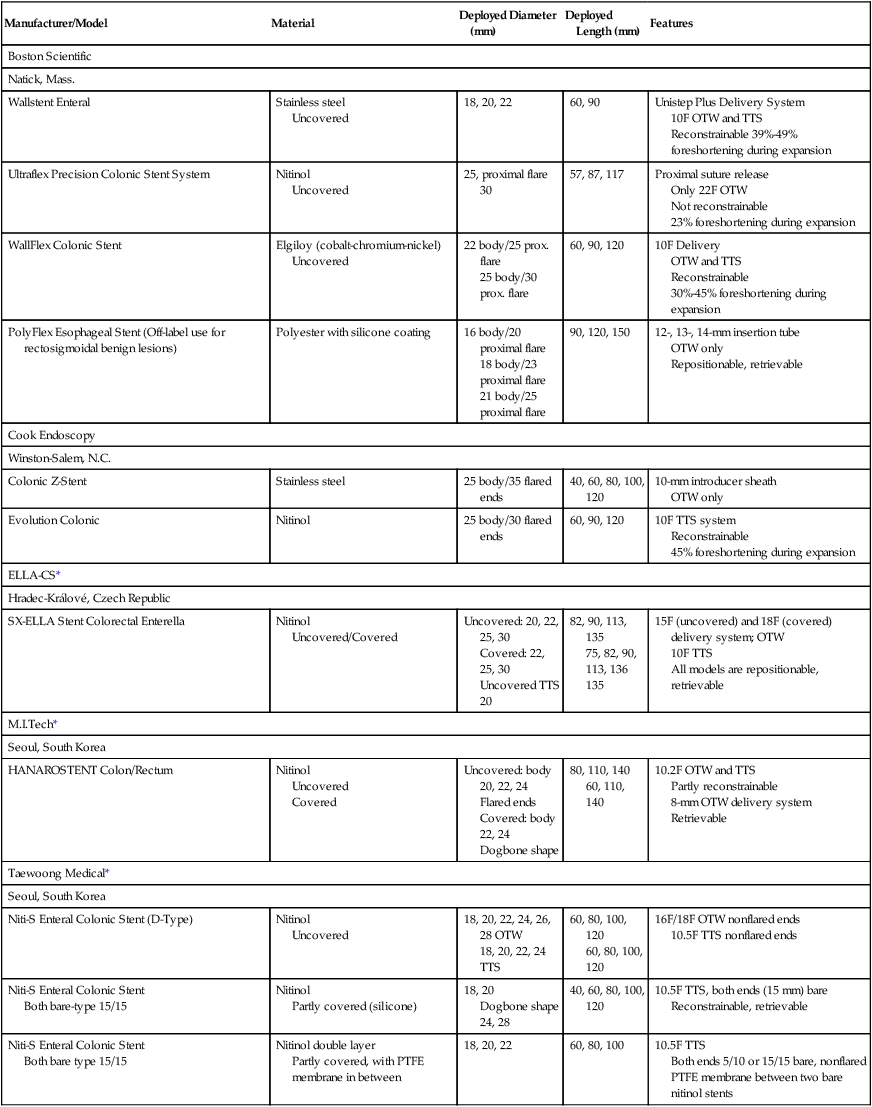
Manufacturer/Model
Material
Deployed Diameter (mm)
Deployed Length (mm)
Features
Boston Scientific
Natick, Mass.
Wallstent Enteral
Stainless steel
Uncovered
18, 20, 22
60, 90
Unistep Plus Delivery System
10F OTW and TTS
Reconstrainable 39%-49% foreshortening during expansion
Ultraflex Precision Colonic Stent System
Nitinol
Uncovered
25, proximal flare 30
57, 87, 117
Proximal suture release
Only 22F OTW
Not reconstrainable
23% foreshortening during expansion
WallFlex Colonic Stent
Elgiloy (cobalt-chromium-nickel)
Uncovered
22 body/25 prox. flare
25 body/30 prox. flare
60, 90, 120
10F Delivery
OTW and TTS
Reconstrainable
30%-45% foreshortening during expansion
PolyFlex Esophageal Stent (Off-label use for rectosigmoidal benign lesions)
Polyester with silicone coating
16 body/20 proximal flare
18 body/23 proximal flare
21 body/25 proximal flare
90, 120, 150
12-, 13-, 14-mm insertion tube
OTW only
Repositionable, retrievable
Cook Endoscopy
Winston-Salem, N.C.
Colonic Z-Stent
Stainless steel
25 body/35 flared ends
40, 60, 80, 100, 120
10-mm introducer sheath
OTW only
Evolution Colonic
Nitinol
25 body/30 flared ends
60, 90, 120
10F TTS system
Reconstrainable
45% foreshortening during expansion
ELLA-CS*
Hradec-Králové, Czech Republic
SX-ELLA Stent Colorectal Enterella
Nitinol
Uncovered/Covered
Uncovered: 20, 22, 25, 30
Covered: 22, 25, 30
Uncovered TTS 20
82, 90, 113, 135
75, 82, 90, 113, 136
135
15F (uncovered) and 18F (covered) delivery system; OTW
10F TTS
All models are repositionable, retrievable
M.I.Tech*
Seoul, South Korea
HANAROSTENT Colon/Rectum
Nitinol
Uncovered
Covered
Uncovered: body 20, 22, 24
Flared ends
Covered: body 22, 24
Dogbone shape
80, 110, 140
60, 110, 140
10.2F OTW and TTS
Partly reconstrainable
8-mm OTW delivery system
Retrievable
Taewoong Medical*
Seoul, South Korea
Niti-S Enteral Colonic Stent (D-Type)
Nitinol
Uncovered
18, 20, 22, 24, 26, 28 OTW
18, 20, 22, 24 TTS
60, 80, 100, 120
60, 80, 100, 120
16F/18F OTW nonflared ends
10.5F TTS nonflared ends
Niti-S Enteral Colonic Stent
Both bare-type 15/15
Nitinol
Partly covered (silicone)
18, 20
Dogbone shape 24, 28
40, 60, 80, 100, 120
10.5F TTS, both ends (15 mm) bare
Reconstrainable, retrievable
Niti-S Enteral Colonic Stent
Both bare type 15/15
Nitinol double layer
Partly covered, with PTFE membrane in between
18, 20, 22
60, 80, 100
10.5F TTS
Both ends 5/10 or 15/15 bare, nonflared
PTFE membrane between two bare nitinol stents
Technique
Approach
Technical Aspects
Fluoroscopic Guidance/Radiologic Technique (OTW)
Endoscopic-Fluoroscopic Guidance (TTS)
Further Technical Considerations
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Preoperative and Palliative Colonic Stenting