In this article, some specificities of functional magnetic resonance imaging (fMRI) in children (eg, blood-oxygen-level-dependent response and brain maturation, paradigm design, technical issues, feasibility, data analysis) are reviewed, the main knowledge on presurgical cortical mapping in children (motor, language, reading, memory) is summarized, and the emergence of resting state fMRI in presurgical cortical mapping is discussed.
Key points
- •
Blood-oxygen-level-dependent contrast may be negative in babies and infants.
- •
Simple block paradigms are the most robust for clinical applications in children; passive tasks can be used.
- •
Brain plasticity is maximal in children, with a possibility of efficient right organization of language in early left hemisphere lesions.
- •
Contralateral motor reorganization is also possible in early prenatal lesions (persistence of ipsilateral corticospinal tract).
- •
Resting state functional magnetic resonance imaging may be a promising tool for pretherapeutic mapping in children in the future.
Introduction
In children, like in adults, the main (if not only) clinical application of functional magnetic resonance imaging (fMRI) is to provide reliable mapping of eloquent cortices (mostly, motor and language areas), and of their relationship with the planned resection in case of tumor or epilepsy surgery, to select patients, tailor resection, and avoid postoperative deficits. When combined with clinical, neuropsychological and neurophysiologic data, anatomofunctional magnetic resonance (MR) imaging techniques (MR imaging, diffusion tensor imaging [DTI], and fMRI) offer the possibility of a noninvasive presurgical workup, which has tremendous consequences for children’s management not only by reducing the need for invasive techniques but also by making many more children amenable to presurgical exploration, thus affecting patient management. In that context, all methods implemented must reach high sensitivity for detecting activated areas at the individual level, especially in young children. Therefore, most teams use validated tasks amenable to patients with variable abilities, in robust block paradigms, with individual analyses involving adapted statistical methods. Still most needed here are further validation and standardization of the whole process across clinical teams.
The mere comparison between the explosive number of fMRI studies in adults and the still limited studies in children, especially in clinical applications, highlights the intrinsic difficulties of studying children. Recently, the possibility of studying brain functional connectivity with fMRI during rest (resting state functional connectivity MR imaging) may make it possible to overcome several of those limitations in the future, to assess the development of neuronal networks from birth on, in health and in disease, and to map eloquent cortex before surgery even in uncooperative or deficient patients.
In this article, some specificities of fMRI in children (eg, blood-oxygen-level-dependent [BOLD] response and brain maturation, paradigm design, technical issues, feasibility, data analysis) are reviewed, then the main knowledge on presurgical cortical mapping in children (motor, language, reading, memory) is summarized, and the emergence of resting state fMRI in presurgical cortical mapping is discussed.
Introduction
In children, like in adults, the main (if not only) clinical application of functional magnetic resonance imaging (fMRI) is to provide reliable mapping of eloquent cortices (mostly, motor and language areas), and of their relationship with the planned resection in case of tumor or epilepsy surgery, to select patients, tailor resection, and avoid postoperative deficits. When combined with clinical, neuropsychological and neurophysiologic data, anatomofunctional magnetic resonance (MR) imaging techniques (MR imaging, diffusion tensor imaging [DTI], and fMRI) offer the possibility of a noninvasive presurgical workup, which has tremendous consequences for children’s management not only by reducing the need for invasive techniques but also by making many more children amenable to presurgical exploration, thus affecting patient management. In that context, all methods implemented must reach high sensitivity for detecting activated areas at the individual level, especially in young children. Therefore, most teams use validated tasks amenable to patients with variable abilities, in robust block paradigms, with individual analyses involving adapted statistical methods. Still most needed here are further validation and standardization of the whole process across clinical teams.
The mere comparison between the explosive number of fMRI studies in adults and the still limited studies in children, especially in clinical applications, highlights the intrinsic difficulties of studying children. Recently, the possibility of studying brain functional connectivity with fMRI during rest (resting state functional connectivity MR imaging) may make it possible to overcome several of those limitations in the future, to assess the development of neuronal networks from birth on, in health and in disease, and to map eloquent cortex before surgery even in uncooperative or deficient patients.
In this article, some specificities of fMRI in children (eg, blood-oxygen-level-dependent [BOLD] response and brain maturation, paradigm design, technical issues, feasibility, data analysis) are reviewed, then the main knowledge on presurgical cortical mapping in children (motor, language, reading, memory) is summarized, and the emergence of resting state fMRI in presurgical cortical mapping is discussed.
How to perform functional MR imaging in children
Blood-Oxygen-Level-Dependent Contrast in the Developing Brain
The developing brain is characterized by a succession of progressive and regressive events, with intense synaptic growth from birth on leading to synaptic overproduction and redundancy in the primary school years, and then, slow progressive synaptic pruning with stabilization of efficient networks during adolescence and young adulthood. This triphasic process occurs earlier in primary systems, and later into the second decade for the associative networks, and is accompanied by changes in glucose consumption and blood flow.
In the recent years, fMRI has emerged as a unique tool to study the exquisite plasticity of the immature brain, which sustains both normal learning and memory acquisition, and recovery after a focal insult or abnormality with an incomparably better functional outcome compared with adults with similar condition. The general pattern of functional maturation of a specific network has been shown as regional specialization of activated clusters with age, starting from a more widespread activation in earlier ages, and associating progressive and regressive changes in different regions. These focal changes are associated with changes in short-range and long-range connectivity, as recently discovered by functional connectivity studies (see later discussion).
These combined biological events in immature networks, associated with changes in vascular reactivity during neuronal firing, may contribute to the negative BOLD response during visual and sensorimotor stimulations described in neonates and infants. However, part of this negative response might also be attributed to sedation, which is commonly used at this age. Beyond the first weeks of life, BOLD hemodynamic response is stable across ages, although with possible increase in amplitude until adulthood, and some variations across tasks.
Which Paradigm Design?
Block or event?
So far, all fMRI clinical applications in children have used block paradigms, in which the patient performs the tasks repeatedly over activation and reference periods (usually 20 to 40 seconds each), repeated several times in a single trial. This approach is the most robust and reproducible, because of good statistical power per unit time, and therefore is used in clinical applications such as individual presurgical motor or language mapping. It can also be usefully applied in clinical research programs in which children are to be pooled in groups for comparing activation differences according to a clinical marker.
More sophisticated single-event paradigms, which allow monitoring brain response during processing of a single stimulus, may prove useful in patients, because they avoid using a control task (thus reducing a possible confounding factor), they permit accounting for interstimulus response variability, and they offer extended possibilities of experimental designs eg, in memory studies. However, their implementation remains difficult in clinical environments (especially in children), because of long acquisition times, lower sensitivity, large data volumes, and the need for customized and time-consuming analyses.
Choosing tasks adapted to the child’s age and performance
The most critical part of fMRI studies resides in the choice of activation and reference tasks, because data analysis most often relies on cognitive subtraction (ie, the resulting activated areas are believed to sustain the components that are involved in the activated state but not in the reference one). For example, the comparison of an auditorily cued semantic decision task and a simple tone discrimination task shows mainly regions involved in semantic processes. On the other hand, the comparison of a more global language task (eg, sentence generation to a given noun) compared with simple rest shows a larger functional network, which includes numerous modules of receptive and expressive language (phonemic discrimination, phonology, lexical and semantic processing, syntax, verbal working memory and prearticulatory processing). In motor paradigms, global hand movements like grasping or clutching compared with rest show the hand primary sensorimotor region with a few associated regions (supplementary sensorimotor area), whereas alternating complex sequential finger movements activates a larger network, including the premotor cortex.
fMRI experimental constraints are particularly demanding for children, because paradigms are designed in a rigid manner, according to a priori models of BOLD contrast time course. In addition, the intrinsically low BOLD contrast/noise ratio requires the repetition of events to gain statistical power. In that context, task complexity is critical in children, in terms of cognitive/attentional demands. According to the age and cognitive level of the patients, block paradigms with simple tasks are most often suited, such as, for example, alternating hand movements with rest for motor mapping. Tasks can almost always be adapted to the performance level (even in motor paradigms, from complex sequential finger tapping to simple grasp movement, or passive wrist flexion-extension). In cognitive clinical studies, such as language studies, tasks with explicit demands are most often used, and adapted to the patient’s abilities and performance. In children, this means using tasks adapted from age-validated neuropsychological tests and appropriately testing them before MR imaging.
Strictly passive tasks can also be used in particular cases, such as passive movements in patients with motor deficits, or presentation of auditory stimuli in neonates, and asleep infants and toddlers. Although these tasks may show interesting activation often grossly comparable with that of active ones, they may not provide the same level of functional assessment in cognitive studies. Sedation can be occasionally used to assess basic functions for clinical purposes (eg, auditory stimulation before cochlear implantation, motor mapping ), but with a careful choice of anesthetic drugs and dosages.
The use of rest as a reference task is being debated because of the uncertainty regarding what children do while resting. In all cases, giving clues to the child on rest instruction (eg, listening to the scanner noise, concentrating on their own breathing) may help them to comply. On the one hand, it has the advantage of simplicity for very young or disabled children, who have difficulties in rapidly alternating different tasks. On the other hand, children may involuntarily not stop the activation task (resulting in falsely negative results, especially in language tasks), and there are no means of controlling ongoing cognitive activity during rest. Some experimenters therefore use very simple task like tone listening as references in children with sufficient mental flexibility.
Multiple tasks are required when testing complex cognitive functions such as language to increase the robustness of lateralization assessment by combining the different tasks and to show the whole network. In some instances, lateralization may vary according to the type of task, either as a normal pattern (eg, left semantic vs right prosody) or related to mixed language representation secondary to left focal epilepsy or lesion (eg, with left dominance for expressive language and right dominance for receptive one) (see later discussion).
Controlling performances
Monitoring of task performance is desirable for fully analyzing and interpreting activation maps, because the latter reflect what has been done during scanning. This analysis may be obtained by monitoring responses of the patient pressing on joystick or buttons. In case of unilateral responses, balancing the side of the responding hand may be useful to avoid systematic bias in brain activation. However, this right-left balance may complicate the paradigm for young children not fully acquainted with their own right and left sides. In motor studies, because BOLD signal depends on both movement frequency and strength, these parameters may be controlled for by cuing movements with a metronome, and monitoring them by video recordings, buttons, and so forth. However, in language fMRI studies, most tasks are performed silently, producing similar activation to overt ones, but avoiding artifacts caused by face movements. Not only does this situation preclude any performance control but it may also not be amenable to deficient children. Some experimenters have used oral responses (with online response recording), associated with adapted MR sequences with no image acquisition during the response interval to avoid articulation-related motion artifacts, taking benefit from the delayed hemodynamic response. Eye-tracking devices are being increasingly used to monitor eye movements during cognitive tasks, but the experimental setup is demanding for children and remains largely beyond the possibilities of a clinical environment.
Technical Issues
Hardware
High field MR imaging is being considered by the US Food and Drug Administration as minimal risk procedure up to 8 T for adults and children, and up to 4 T for neonates younger than 1 month. Three-Tesla fMRI has become standard in babies and children as in adults, including in healthy individuals for research protocols. The increased sensitivity of BOLD contrast at higher field strength can be used to either shorten acquisition time or increase spatial resolution to improve localization of activated clusters, which can be valuable in young children with small heads, all while maintaining adequate signal-to-noise ratio.
Multichannel coils with parallel imaging further improve the signal-to-noise ratio but make it even more critical to use coils adapted to the head size. In neonates and infants, smaller coils such as knee coils provide better signal and improve the sensitivity of fMRI. MR-compatible incubators are needed to prevent hypothermia in neonates (especially premature babies), but they are expensive and cumbersome.
Acoustic noise
One issue often neglected in fMRI is the acoustic noise created by Lorentzian forces secondary to gradient switching in echo planar images (EPI) (in functional imaging as well as in DTI). Not only may noise prevent children from remaining still because of anxiety or difficulty in sleeping but the risk of acoustic trauma must also not be underestimated, because functional MR imaging sequences often reach 110-db levels at peak frequencies. Because noise level depends on multiple sequence parameters (eg, type of sequence, spatial and temporal resolutions, parallel imaging), acoustic measurements should be ideally performed for each sequence, whenever possible. Careful prevention must always be undertaken, including in asleep or sedated infants and children, with a variety of devices (eg, earplugs, headphones, foams). Some manufacturers offer hardware options reducing acoustic noise of various sequences by modifying gradients, shape, and strength.
Temporal and spatial resolution
Temporal resolution of fMRI mainly depends on the shape of the hemodynamic response, which is comparable with adults after the first few months of life (see earlier discussion). Shortening repetition time from standard 3 to 5 seconds to 2 to 2.5 seconds provides better sampling of subtle variations of the hemodynamic response and increases statistical power (beware of acoustic noise).
Spatial resolution can strongly benefit from higher fields, and it is standard practice to acquire 3-T EPI data with 3 × 3 × 3 mm 3 voxels. Although this nominal resolution is not clearly reflected in the results because of numerous steps of spatial filtering, it permits better localization of activated clusters in small anatomic regions by reducing partial volume effects. On the other hand, motion artifacts are more conspicuous and problematic in highly resolved scans.
Real-time fMRI may be of particular value in clinical studies in children, because it provides continuous monitoring of the acquisition, by reconstructing and analyzing the images online, and providing a constant update of the quality of the functional study. This factor is especially relevant when paradigms are being kept simple, with reasonable data sets and standard statistical analyses. However, this might prove a challenge in more sophisticated studies with event-related paradigms, large data sets, and analyses requiring heavy postprocessing.
Feasibility of Fuctional MR Imaging in Children
Cooperation
In fMRI activation studies, the child’s cooperation is critical. However, MR imagers remain child unfriendly, and strict immobility is mandatory to avoid motion artifacts. Obtaining compliance to the tasks is therefore a challenge in young or deficient children and requires extra time and resources, with at best a dedicated visit before the study with the child and the parents. This visit with the experimenter(s) who will perform the fMRI study (eg, radiologist, neurologist, neuropsychologist) is necessary to show the child the imager and explain the tasks, to train them to remain still in a mock scanner if available, and to practice the fMRI tasks. It also makes it possible to adapt the paradigm in case of poor compliance to optimize feasibility of clinical studies, and sometimes, to cancel the study, when sufficient compliance cannot be obtained, thus optimizing scanner occupancy. During scanning, many adaptations can be implemented to improve the child’s comfort and quietness, such as having the experimenter and the parents in the magnet room, interacting often with the child through intercom, playing movies on a screen during anatomic scans, monitoring the child’s movements through an MR-compatible camcorder and providing them with feedback during scanning. In all cases, good head immobilization is necessary to discourage movements. Providing the child with visual inputs also decreases head motion artifacts.
Scan duration
Statistical power depends directly on the number of scan repetitions and thus, on scanning duration. On the one hand, paradigms are to be made long enough to obtain reliable contrast to noise; on the other hand, attentional resources and compliance of children cannot be maintained during scans that are too long. Higher field acquisitions (3 T) do contribute to alleviate these constraints. Still, unlike in adults, in whom 10-minute to 15-minute runs are commonly acquired, in most children, the whole acquisition is preferably segmented in shorter runs of 2 to 4 minutes. It is advisable to repeat similar runs during a single session, given the high rate of poor-quality data in younger children. A whole study (including anatomic images, diffusion-weighted images with a sufficient number of directions to perform reliable tractography, and fMRI) must be completed in 20 minutes in babies or poorly compliant children, and in a maximum of 1 hour in older school-age children with no significant cognitive or behavioral impairments. In some instances, additional sequences may be needed (eg, gadolinium-enhanced perfusion MR, MR angiography, arterial spin labeling). Acquisition may then be split or repeated in 2 or more sessions separated by a break, even on separate days, because there are no limitations, given the absence of known side effects. In that case, images from different sessions must be coregistered during analysis.
Cooperation can be obtained for adapted paradigms from children with a developmental age of around 5 to 6 years or IQs around 60, if there are no behavioral disorders. Passive tasks can be used in sleeping or quiet neonates, infants, and children (either with or without sedation), using receptive language tasks, sensory stimulations, and so forth. Overall, the attrition rate of fMRI studies in children is higher than in adults, especially in activation studies.
Data Analysis
Head motion remains a critical limiting factor, especially in uncooperative, young, or debilitated patients, and is more frequent and pronounced in boys than in girls. The use of dedicated registration algorithms is most often necessary, but the choice of registration method is empirical, depending on the type and amplitude of movements, and does not significantly influence the results. Motion parameters can be introduced as regressors of noninterest in the analysis to reduce variance. Some algorithms are based on data interpolation to replace heavily corrupt images; others take into account discarded, and thus missing, data. Overall, the resulting corrected images must be checked carefully, because many registered data may be discarded because of insufficient correction.
Statistical analysis of pediatric data sets follows the same rules and constraints as in adults and strongly depends on the goal of the study: in presurgical studies of patients, the main goal is to optimize at the individual level the sensitivity of the detection of activated areas; resection of these areas results in postoperative deficits, which may depend on multiple factors (eg, age, efficiency, attention, disease, medications). In such circumstances, testing multiple thresholds and taking into account the performance level are likely to increase the sensitivity of detection of activated networks, especially in children. On the other hand, a threshold that is too lenient leads to widespread activation, which may overestimate the risk of lesioning an eloquent cortex and, thus, lead to unjustified denial of surgery. Overall, in presurgical studies, thresholding and interpretation of activation maps still depend heavily on the expertise of the investigator, especially for data sets containing residual motion artifacts.
Mapping eloquent cortex in focal epilepsies and brain tumors in children
Clinical fMRI activation studies in children are basically limited to presurgical mapping, to select patients for surgery and avoid postoperative deficits, by planning the resection according to the spatial relationship between eloquent cortices and the epileptogenic zone or the tumor. In addition, DTI can be obtained during the same MR examination, to show the anatomic connectivity of involved regions, by tracking the main fascicles (eg, corticospinal tracts, arcuate and uncinate fasciculi). When combined with clinical, neuropsychological, and neurophysiologic data, fMRI offers the possibility of reducing the need for invasive techniques and extends the range of patients amenable to presurgical explorations. However, fMRI highlights regions that are activated by, but not always critical to, the tasks (ie, resection would not necessary lead to functional deficit), depending on the type of contrast that has been designed in the paradigm. This situation is to be contrasted with electrostimulation methods, in which transient induced functional deficits are supposed to reproduce those that result from the (undesirable) resection of the stimulated region. However, numerous studies have shown the good correspondence of both methods when appropriate methodologies are being used, and their complementarities, in children as in adults.
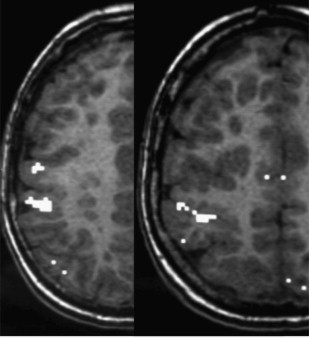
fMRI has also shown that some malformations of the cortical development (eg, heterotopias, polymicrogyrias) may retain functional cortical organization (eg, vision, motor, language) with a risk of postoperative deficit in case of a resection of the malformed cortex ( Fig. 1 ). By contrast, it seems that Taylor-type focal cortical dysplasias may not retain functional activity within the area containing balloon cells (hypersignal on fluid-attenuated inversion recovery or T2 images), although this is debated.