Fig. 1
CT head showing traumatic SAH
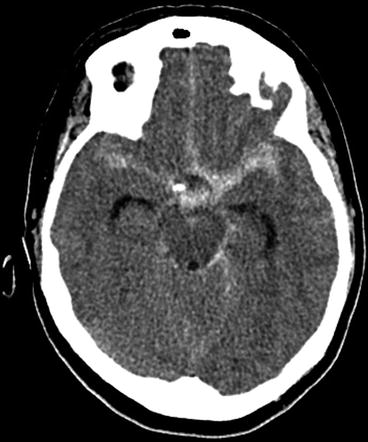
Fig. 2
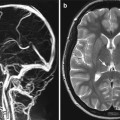
Unenhanced axial CT brain showing central aneurismal SAH
Spontaneous SAH is most often (85 %) caused by a ruptured aneurysm. More rare causes include ruptured arteriovenous malformations at about 8 % and dural arteriovenous fistula at less than 1 %. This leaves about 5–10 % of cases in which no cause is found, and a venous rupture causing a perimesencephalic bleed is suspected. The importance of this latter group is that their risk of rebleeding is low and no definitive treatment is required. Due to the high rebleed rate in aneurismal SAH, urgent investigation and treatment has become the routine following the publication of the International Study of Aneurysm Treatment (ISAT), and this has led to the subsequent development of interventional neuroradiology and endovascular neurosurgery. Aneurysms can present with mass effect (Fig. 3a, b) or rarely with thromboembolic complication of the intraluminal thrombus.
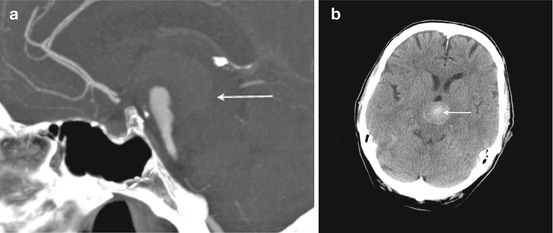

Fig. 3
(a) Sagittal maximum intensity projection (MIP) of a contrast-enhanced CT angiogram showing the mass effect of a giant basilar tip aneurysm that is substantially thrombosed. (b) Unenhanced axial CT brain showing the same aneurysm with high attenuation thrombus demonstrated near the aneurysm dome (arrow)
Risk Factors
Smoking
Hypertension
Alcohol abuse
Positive family history
Collagen dysfunction diseases
Female predominance over 30 years of age
Investigations
CT to Show SAH
Sensitivity for SAH at 24 h = 98 %
Sensitivity for SAH at 5 days = 70 %
Sensitivity for SAH at 7 days = 50 %
Lumbar Puncture (LP) to Show SAH in CT-Negative Cases and Delayed Presentation
Must be processed (spun down) by the lab immediately to decrease the false positive rate
98–100 % sensitive from 9 h to 2 weeks
70 % sensitive at 3 weeks
40 % sensitive at 4 weeks
Magnetic Resonance Imaging (MRI)
T1, FLAIR, and gradient echo-/susceptibility-weighted imaging (SWI) sequences to look for blood
Computed Tomographic Angiography (CTA)
May miss small aneurysms at the skull base
Digital Subtraction Angiography (DSA)
Gold Standard with the best spatial and temporal resolution but comes at a cost of 1/2000 permanent neurological deficit
Endovascular Aneurysm Treatment (EVT)
Consent is the cornerstone of the patient–doctor relationship. It is important to cover:
Indications
Primary objective is to reduce the risk of rebleeding from 2 % per day to 0.2 % per year.
Secondary objectives are to allow aggressive hypertensive treatment for delayed cerebral ischemia.
Prevent recurrence.
Risks
5 % groin hematoma
2–4 % new neurological deficit (local data/ISAT)
Alternatives
Neurosurgical clipping
Conservative treatment
Special techniques
Preoperative Medication
Dual antiplatelet medication, used in the setting of unruptured aneurysms
World Health Organization (WHO) Checklist
Confirmation of the correct patient and procedure
Allergies
Risk factors for bleeding
Any special requirements
Review previous imaging
Performed under general anesthesia
Groin Puncture: Right, Left, or Both
Right normally, as close to the operator.
Left if indwelling lines already in the right or difficult access due to previously deployed stent, scar tissue, peripheral vascular disease, or bypass.
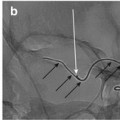
Both, check if angiography of both internal carotid artery (ICA) is needed e.g. while treating an anterior communicating artery complex aneurysm (Fig. 4a, b).
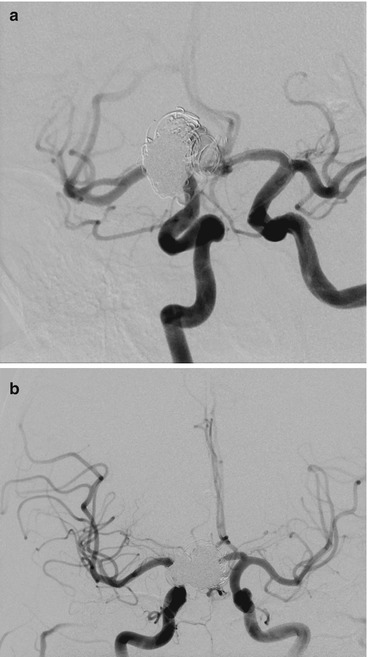
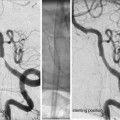
Fig. 4
(a, b) Shows dual ICA cannulation to allow contra lateral ACA control while coiling proceeds
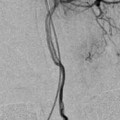
Diagnostic Angiography
Target aneurysm harboring vessel first with an AP and lateral whole head with which to compare the pre- and post-embolization images looking for distal embolic events followed by a 3-dimensional (3-D) rotational angiogram (Fig. 5a–d) for the selection of working projections and measurement of the aneurysm neck and dome. The remaining vessels can then be interrogated while processing and manipulating the volume data.
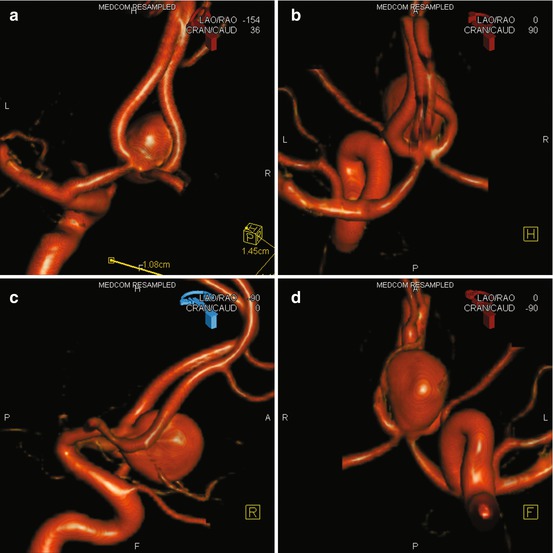
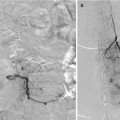
Fig. 5
(a–d) Showing a 3-D rotational angiogram of an ACom aneurysm viewed from behind, above, laterally, and below, respectively
Guide Catheter
Stable safe position just below the skull base in the majority of cases on a heparin containing flush bag.
Intra-procedure Medication