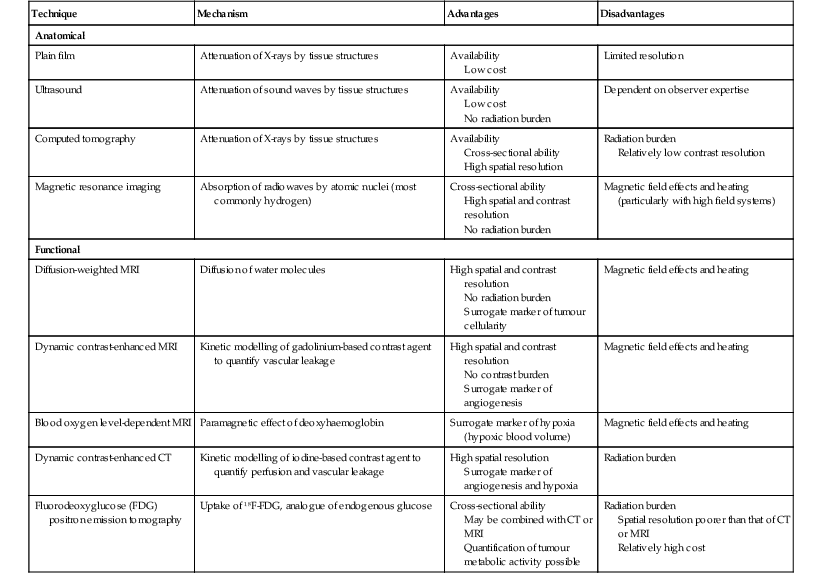
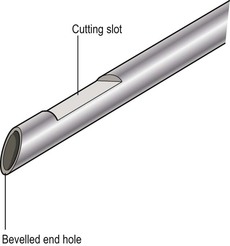
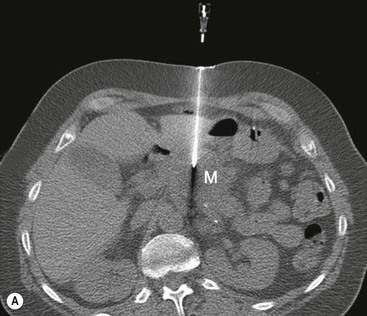
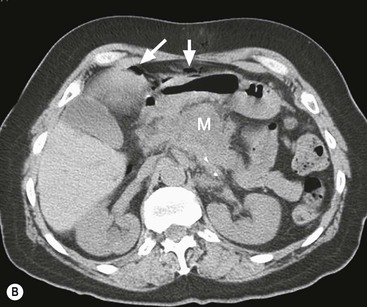
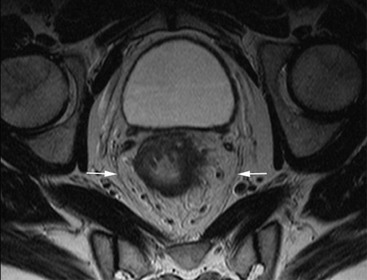
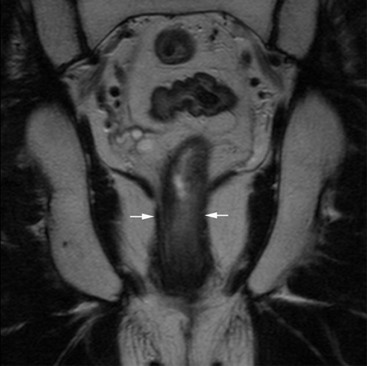
David MacVicar, Vicky Goh Cancer is one of the major causes of death in the Western world, costing an estimated US$125 billion in 2010. There were 1.6 million projected new cancer cases and 577,190 cancer deaths in the USA alone in 2012.1 The incidence of cancer is also increasing in developing countries, related to factors such as smoking, and shifts towards a more Western lifestyle. The commonest cancers include lung, bowel, breast and prostate cancers. In recent years there have been major advances in the approach to the assessment and treatment of cancer: for example, the introduction of screening, genomic testing and multimodality treatment, including: The subspeciality of oncological imaging has evolved in tandem with this. Oncological imaging now forms a significant proportion of the workload of a radiology department.2 Imaging plays a major role at different stages along the patient pathway. Cross-sectional imaging is used widely for diagnosis, staging, assessment of treatment response and surveillance. A variety of anatomical and functional imaging techniques are available in clinical practice currently. High-resolution cross-sectional imaging techniques such as computed tomography (CT) and magnetic resonance imaging (MRI), which allow the whole body to be imaged with high accuracy, remain the mainstay of imaging practice. However, physiologically based functional imaging techniques that assess different aspects of tumour biology such as diffusion-weighted MRI (water diffusion; a surrogate of tissue cellularity) and dynamic contrast-enhanced CT or MRI (tumour perfusion and vascular leakage; a surrogate of angiogenesis) have been applied increasingly in clinical practice to improve tumour detection, staging and response assessment. Molecular imaging techniques such as positron emission tomography (PET) provide more targeted imaging of tumour physiology and biology, with excellent anatomical localisation as hybrid imaging modalities (PET/CT and PET/MRI). While 18F-fluorodeoxyglucose (FDG), an analogue of glucose, remains the commonest radio-labelled tracer in clinical use, allowing assessment of glucose metabolism, other tracers including 18F-fluorothymidine (FLT), 11C-choline, 18F-misonidazole (FMISO), 18F-FAZA, 61C- or 64Cu-ATSM and 11C-acetate provide relevant information on tumour proliferation, hypoxia and lipogenesis, respectively. Each imaging modality has advantages and disadvantages (Table 68-1) and the ‘best’ imaging strategy will depend on the tumour type, tumour site, clinical indication (diagnosis, staging, treatment response assessment or surveillance), and availability and cost of the imaging technique. A consequence of these advances in assessment and treatment has been improvements in patient outcome: this is especially so for early-stage disease, although survival for patients with advanced disease remains relatively poor. Another has been the recognition of the need for local, regional and national changes in the organisational aspects of cancer care. Typically in the United Kingdom, all new presentations of cancer are now assessed at a multidisciplinary meeting, and managed by a multidisciplinary team of specialists (including doctors (surgeons, medical and clinical oncologists, physicians, radiologists, pathologists), nurses, dieticians and physiotherapists) that are experienced in their cancer type in order to optimise clinical management. For a radiologist, this provides the opportunity to ensure the most suitable investigations are performed in a timely fashion at diagnosis, during treatment and in subsequent follow-up. This chapter will introduce key concepts in the imaging of patients with cancer. The role of imaging in diagnosis, staging, response assessment and surveillance will be described. In the majority of cases, a patient will present with symptoms and signs related to the cancer, and appropriate investigations will be arranged, including imaging. Usually there are only a few diagnoses that can be made with confidence from imaging characteristics: for example, an ovarian dermoid, or other fat-containing tumours, and lesions that are obviously cystic. In most cases there is a differential diagnosis, requiring pathological confirmation of the diagnosis. Confirmation of a diagnosis may be undertaken using a variety of techniques, including cytological examination of fine needle aspiration samples, tumour specimens from automatic cutting needles and surgical biopsies (Figs. 68-1 and 68-2). Core biopsies yield a higher tumour volume than fine needle aspiration, and may be more suited for tumour biomarker analysis, often required by clinical trials. Certain principles should be followed when needle aspiration or percutaneous core biopsy is being planned: Several types of cutting needles may be used for core biopsy. The Tru-Cut needle has a central trocar that makes the initial movement forward, exposing a slot. The sheath is then advanced over the slot, cutting a piece of tissue into the slot, and the entire apparatus is withdrawn, with a core specimen contained in the slot. Coaxial systems enable multiple tumour cores to be obtained via a single puncture site. The principle involves placement of a needle from which the sharpened trocar is removed to leave a hollow unsharpened cannula in the mass for biopsy. An automatic cutting needle can then be placed down the cannula. Several cores can then be obtained, and different parts of the lesion may be sampled by angling the cannula slightly in a variety of directions. Since only one puncture is made of the skin and intervening tissue planes, more tissue is obtained without increasing the risk of track seeding. In some circumstances percutaneous needle techniques should not be used to avoid malignant seeding of the percutaneous biopsy track. Once the diagnosis of cancer has been confirmed, it is important for the subsequent management of a patient to stage the tumour, i.e. to assess the locoregional extent of the tumour as well as the presence/absence of distant metastatic disease. The aims of a staging system are: • To give some indication of the likely prognosis; • To assist in the evaluation of results of treatment; • To enable exchange of information between cancer treatment centres; and • To contribute to the continuing investigation of human cancer. Staging systems describe the anatomical extent of a tumour, and provide highly relevant information to guide appropriate therapy at diagnosis, although decision-making will be influenced also by other factors, including the histological grade of the tumour, its expected biological behaviour and the age and general fitness of a patient.3 While clinical examination continues to have a significant role in the initial assessment of patients, imaging has a major role to play in the staging of cancer. An ideal staging system should be simple, precise, consistent and applicable to all clinical circumstances in oncology, and convey some prognostic information to facilitate best practice. Over the years, many staging systems have borne the name of eminent doctors (e.g. the Robson staging classification of renal tumours or Dukes’ staging classification of colorectal cancer), institutions (e.g. the Royal Marsden Hospital staging classification for testicular germ cell tumours) or organisations (e.g. the Fédération Internationale de Gynécologie et d’Obstétrique (FIGO) classification systems for cervical, uterine and other gynaecological neoplasms). More recently, the tumour–node–metastasis (TNM system), espoused by the American Joint Committee on Cancer (AJCC) and the Union Internationale Contre le Cancer (UICC), has been adopted widely. The TNM classification of malignant tumours and the AJCC cancer staging handbook are now in their seventh editions.4 The system was originally devised by Pierre Denoix in the 1940s and has been modified over the subsequent decades. The ‘T’ category entails evaluation of local tumour extent. The ‘N’ category entails evaluation of nodal involvement. The ‘M’ category entails evaluation of disease at distant sites. The ‘T’ category, which may have the prefix ‘c’ to indicate ‘clinical’ staging, although this is frequently omitted, has several standard forms of notation: Tx indicates that primary tumour cannot be assessed; Tis indicates in situ disease with no evidence of invasion; T0 indicates no visible evidence of primary tumour; T1–T4 indicates increasing degrees of local tumour extent. These divisions may be adapted with the addition of subdivisions indicated by letters (e.g. ‘a’ or ‘b’) for greater flexibility in different tumour types. Although staging of the primary from T1 to T4 follows broad principles and there are some similarities between tumour types, refinements and adaptations for individual tumours are needed usually. The ‘N’ category has similar notation. Direct spread of the primary tumour into an adjacent lymph node is classified as nodal spread. Nx is where regional lymph nodes cannot be assessed, N0 is where no regional lymph node metastases are present and N1, N2 and N3 indicate increasing involvement of regional lymph nodes. Likewise, these divisions may include subdivisions indicated by letters (e.g. ‘a’ or ‘b’). The‘M’ category assesses distant metastasis where Mx indicates that distant metastasis cannot be assessed, M0 indicates there are no distant metastases and M1 indicates the presence of distant metastasis. The category M1 may be further specified indicating which organs are involved. For example, PUL indicates pulmonary metastases, OSS indicates osseous metastases and HEP indicates hepatic metastases. Again, subdivisions may be indicated by letters (e.g. ‘a’ or ‘b’). All tumours must be confirmed pathologically. A number of general rules apply when using the clinical TNM staging system. Clinical stage is assigned by physical examination, imaging and other relevant investigations, but may be amended as pathological information becomes available, and given the prefix pTNM stage where microscopic extent of disease is known. If there is doubt what stage should be assigned, the lower category should be used. Therefore, an imaging investigation that is suspicious but not diagnostic of spread to the pelvic sidewall will be disregarded unless supplemented by further imaging or confirmation by histopathology. Once assigned, the pre-treatment TNM stage is recorded in the patient’s records and remains unchanged through subsequent treatment. For multiple synchronous primary tumours, the tumour with the highest ‘T’ category is used for staging purposes. For synchronous primary tumours arising in paired organs, each tumour should be assigned a separate TNM stage. In modern usage the TNM system has the advantages of clarity of communication, but is complex. This has led to a further system of stage grouping, which is published within the AJCC system. Stage groups of 0–4 are assigned as tumour becomes more extensive and widespread. National bodies, such as the Royal College of Radiologists in the United Kingdom, have published recommendations or guidelines on the choice of staging investigation (CT, MRI or PET/CT), recognising that local availability of advanced imaging techniques and the preference and experience of individual radiologists are important considerations.5 A potential effect of advances in imaging technology over time is stage migration, e.g. via improvements in tumour detection, resulting in upstaging, and artefactual improvement in subgroup prognosis, although overall survival will remain stable unless more effective treatment is given. The principles of staging are illustrated in this section, with the following examples of some of the most common cancers. Rectal cancer is a good example of a tumour in which a staging system has evolved and changed over a period of more than 70 years, and technical refinement of surgery over the past 20 years has gone hand-in-hand with increasingly sophisticated imaging of the primary tumour such that imaging is now central to decision-making. It remains true that the vast majority of patients with rectal cancer should be offered surgery, as local symptoms due to growth within the pelvis are associated with severe pain that can be difficult to palliate. However, the timing of surgery and the role of neoadjuvant chemotherapy and radiotherapy depend on the local tumour stage at the time of diagnosis. In 1932, Dukes highlighted the importance of extramural spread in the prediction of local recurrence and survival. He also observed that lymph node invasion was present in 14% of patients with tumours confined to the bowel wall and 43% of patients with tumours extending beyond the serosa.6 A number of other prognostic indicators have subsequently been identified, including the pattern of local spread (a well-circumscribed margin implies a better prognosis than a widely infiltrated tumour with ill-defined borders). Spread beyond the peritoneal membrane results in a high incidence of both local recurrence and transcoelomic dissemination; invasion of extramural veins by tumour and extent and number of tumour-involved lymph nodes are all independent predictors of a poor prognosis. For many years the preferred operation for rectal cancer was synchronous combined abdominoperineal excision of rectum (AP resection). However, total mesorectal excision has become the gold standard since its introduction in the 1970s.7 In this technique, the surgeon dissects from above and finds the mesorectal plane. As the lymphatic vessels and nodes draining the tumour are located throughout the mesorectum, if this is divided or disrupted during surgical excision, spillage of malignant cells may occur and involved nodes may be left in situ. This increases the likelihood of local recurrence, and excision of the rectum is best achieved by dissection down the mesorectal fascia, allowing the mesorectum and rectum to be removed en bloc without disruption of the presacral fascia and the underlying venous plexus. The surgically excised specimen may then be sectioned transversely and the circumferential resection margin (CRM) examined for tumour involvement. The presence of tumour at the extremity of the resection margin is a major prognostic factor.8 In recent years, MRI has been shown to be the investigation of choice in demonstrating spread of tumour within the mesorectum and the mesorectal plane itself (Fig. 68-3). It is possible to demonstrate the relationship of the tumour to the anal canal, indicating the potential for sphincter-preserving surgery (Fig. 68-4). Criteria have been developed in pathologically correlated series for staging of the primary tumour and nodes within the mesorectum. Effectively, MRI can provide a ‘road map’ for the surgeon and studies have shown that the pathological status of the CRM can be predicted.9,10 Other prognostic factors such as extramural venous invasion and peritoneal penetration can be demonstrated. If locally advanced disease with mural penetration is demonstrated, chemotherapy and radiation therapy may be employed to downstage the primary before an attempt at surgery, allowing a more tailored preoperative strategy.
Principles of Oncological Imaging
Introduction
Diagnosis
Primary Diagnosis
Confirmation of Diagnosis
Staging
Staging Systems
Principles of Staging Investigations
Primary Tumour Staging
Rectal Cancer