Chapter 21 Production of X-rays
Chapter contents
21.2 Interactions of electrons with matter 151
21.3 Interactions between electrons from the filament and the outer electrons of the target atoms in the X-ray tube 151
21.4 Interactions between electrons from the filament and the nuclei of the target atoms in the X-ray tube 152
21.5 Inelastic collisions with the electrons of the target atoms – production of characteristic radiation 154
21.1 Aim
The aim of this chapter is to consider the mechanisms by which X-rays are produced at the target of the X-ray tube. The concept of the X-ray spectrum will be introduced and this chapter will act as a foundation for Chapter 22 where the factors affecting the X-ray spectrum will be considered.
21.2 Interactions of electrons with matter
In Chapter 30 we will discuss the construction of the X-ray tube and the functions of its various components. In this chapter we will look in detail at the processes involved at subatomic level in the target of the X-ray tube to allow the production of X-rays.
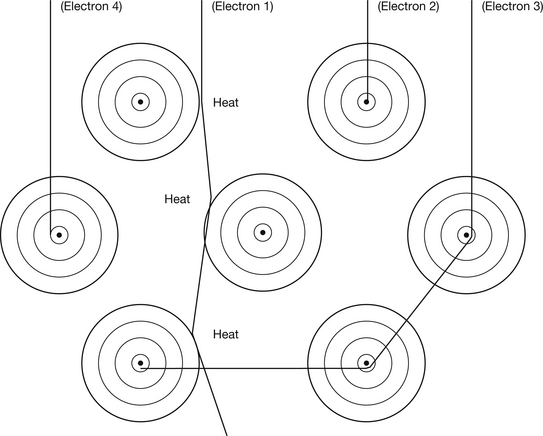
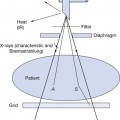
Electrons are produced at the filament of the X-ray tube, accelerated towards the target of the tube and then made to collide with the target atoms. There are a number of ways in which high-energy electrons from the filament of the X-ray tube may lose their energy when they collide with the atoms of the target. These are shown in Figure 21.1:
• Electrons from the filament lose energy because of interactions between them and the outer electrons surrounding the atoms of the target material (see electron 1 in Fig. 21.1).
• Electrons from the filament lose energy because of interactions between them and the nuclei of the atoms of the target material (see electrons 2 and 3 in Fig. 21.1).
• Electrons from the filament lose energy because of interactions between them and individual inner electrons of the target atoms (see electron 4 in Fig. 21.1).
The interactions between electrons and target atoms may be elastic where there is conservation of kinetic energy or inelastic collisions in which kinetic energy is lost (see Section 3.3). It is also important to realise that an electron from the filament may experience many interactions (typically about 1000) before it is brought to rest within 0.25–0.5 mm of the target surface.
21.3 Interactions between electrons from the filament and the outer electrons of the target atoms in the x-ray tube
As you will see in Chapter 30, the main material of the X-ray tube target is tungsten, which has an atomic number of 74. Thus each target atom is surrounded by 74 electrons in various orbitals. These electrons have the effect of deflecting the approaching electron from the filament from their original path because of the electrostatic repulsion between them and the filament electron. Such deflections from the electron’s original path are small and so the loss of kinetic energy is small. The kinetic energy lost by the electron is emitted as a photon of electromagnetic radiation. The energy of this photon is such that it falls into the infrared part of the spectrum and so heat is produced in the target material. If we consider the statistical probability of this process occurring rather than the other two processes identified in Section 21.2, we can see that it has a very high probability of occurring.
21.4 Interactions between electrons from the filament and the nuclei of the target atoms in the x-ray tube
21.4.1 Elastic collisions with the nuclei
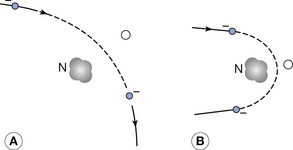
As shown in Figure 21.2, the closer the electron travels to the nucleus, the more it is deflected from its original path by the attraction of the positive nucleus. Because the mass of the electron is so tiny compared to that of the nucleus, the amount of energy it transfers to it is proportionately small.
21.4.2 Inelastic collisions with the nuclei – production of Bremsstrahlung radiation
Classical physics concluded that when a charged particle changed its velocity, this always resulted in the emission of electromagnetic radiation. However, we have already seen that this is not the case, as the electrons in the Bohr model of the atom are continuously changing their velocity (see Sect. 18.4). We have also seen in the previous section that electrons can undergo elastic collisions with the nucleus which result in no electromagnetic radiation being produced. As shown in Figure 21.2, the electron must accelerate towards the nucleus during its deflection but, despite this acceleration, a quantum of electromagnetic radiation is emitted in only a small percentage of cases. When such a quantum is emitted, the kinetic energy of the electron is suddenly reduced by an amount equal to the energy of the quantum, and so the electron is suddenly slowed down or braked.
This is an example of an inelastic interaction since the total kinetic energy of the electron and of the nucleus is not conserved because some energy is removed by the emission of the quantum of radiation. The energy of the quantum may be in the X-ray part of the electromagnetic spectrum and the radiation is known as braking or Bremsstrahlung radiation. The exact energy of this quantum will vary and may be very small if the electron does not lose much energy in its interaction with the nucleus or it may be up to the total kinetic energy of the electron if it is involved in a direct collision with the nucleus (see electron 2 in Fig. 21.1).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree