Key Points
- ▪
CT scanning is currently the de facto test of choice to identify pulmonary embolism.
- ▪
CT scanning should be used within a clinically based cost-conscious algorithm to approach the diagnosis of thromboembolic disease.
- ▪
Imaging features may help distinguish acute from chronic pulmonary emboli—although patients may have both acute and chronic disease.
- ▪
Maximum intensity projection imaging should not be used to evaluate for pulmonary emboli.
- ▪
Other acquired pulmonary artery lesions do occur, such as air embolism of tumor and of medical devices, aneurysms, pseudoaneurysms, mycotic aneurysms, angioinvasion, dissections, aorticopulmonary dissection, traumatic disruptions, compression, calcification, and primary sarcomas.
- ▪
Congenital lesions of the pulmonary artery also may occur, such as central or peripheral stenosis, post-stenotic aneurysms, absence of a pulmonary artery, pulmonary artery slings, and arteriovenous malformations.
- ▪
Pulmonary embolism CT scanning may identify other lesions responsible for chest pain or acute dyspnea presentations.
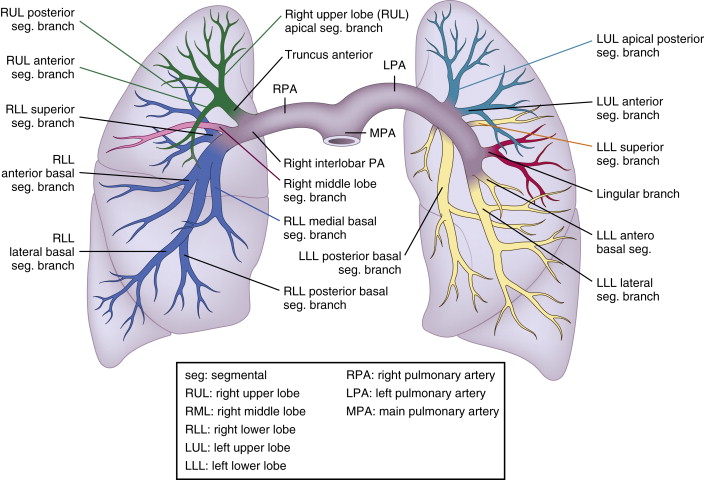
Pulmonary Artery Anatomy
Normally, there are 17 bronchopulmonary segments, any of which may develop an embolism. The main pulmonary artery bifurcates into the right and left main pulmonary arteries. The right main pulmonary artery then trifurcates into three lobar arteries, which divide into segmental arteries (and subsequently into subsegmental arteries, which are not precisely distinguished from the segmental arteries by tomographic imaging):
- □
Upper lobe
- •
S1: apical segment
- •
S2: posterior segment
- •
S3: anterior segment
- •
- □
Middle lobe
- •
S4: lateral segment
- •
S5: medial segment
- •
- □
Lower lobe
- •
S6: superior segment
- •
S7: medial basal segment
- •
S8: anterior basal segment
- •
S9: lateral basal segment
- •
S10: posterobasal segment
- •
The left main pulmonary artery bifurcates into two lobar arteries, which divide into:
- □
Upper lobe
- •
Superior division
- •
S1, S2: apical posterior segment
- •
S3: Anterior segment
- •
- •
Lingular division
- •
S4: superior segment
- •
S5: inferior segment
- •
- •
- □
Lower lobe
- •
S6: superior segment
- •
S7: anteromedial basal segment
- •
S8: lateral basal segment
- •
S9: posterobasal segment
- •
Thus, the pulmonary arterial tree can be described as having:
- □
A main pulmonary artery (trunk)
- □
The left and right main pulmonary arteries
- □
Nine right and eight left segmental pulmonary arteries
- □
Subsegmental pulmonary arteries
See Figure 28-1 .
Pulmonary Thromboembolism
Approximately 700,000 persons per year in North America experience pulmonary embolism (PE). Due to protean presentations, often obfuscated by comorbidity or by surgical issues, delay or missed diagnosis occurs in most cases of PE, causing or contributing to death in an estimated 120,000 patients in the United States alone.
Untreated PE has a recurrence rate of approximately 33%, which entails considerable 3-month mortality: the International Cooperative Pulmonary Embolism Registry reported a rate of 15% to 17%, and historically a rate of 20% to 40% has been reported. In patients in whom PE is identified and treated appropriately, the mortality rate is approximately 8%, versus 30% for untreated patients. It remains strongly suspected that the antemortem detection of PE is considerably suboptimal.
The increasing use of CT scanning to detect pulmonary emboli is associated with an increased detection rate, and declining mortality from pulmonary thromboembolism although whether this is due to the increase in CT scanning or to an overall more aggressive approach to the disorder has not been determined.
The approach to suspected PE entails integration of clinical assessment with or without blood testing for D -dimers into an overall assessment to establish a pre-test probability and decision to proceed to subsequent testing. CT pulmonary angiography is the principal imaging test to assess for suspected pulmonary emboli. CT increasingly affords venography as well, but CT venography is not a proficient test, and its use in the context of suspected PE remains controversial. CT pulmonary angiography contributes to the assessment of medical in-patients and postoperative cases.
Nuclear scintigraphy is less useful in patients with comorbidities and postsurgical cases than it is in ambulatory patients. Pulmonary angiography, the historic “gold standard” examination, retains a role in assessing cases where clinical suspicion remains despite negative testing, and in evaluating cases where there is clinical suspicion and poor CT images. Although CT pulmonary angiography is a robust test, unless poor-quality scans are excluded from analysis, sensitivity and negative predictive value may not always be as strong as is assumed by its users.
For the detection of pulmonary emboli, all pulmonary artery lobar and segmental branches must be investigated. Importantly, there are many possible congenital variants to the pulmonary arterial blood supply.
Determination of the specific anatomic pulmonary artery affected is most readily done with conventional pulmonary angiography, which “lays out” the pulmonary arterial vasculature of one lung. Imaging is intentionally done early to avoid the venous (levo) phase, thereby simplifying the number of vessels depicted and minimizing overlap.
Determination of the specific pulmonary arterial anatomic segment also can be achieved with CT pulmonary angiography, especially if combinations of axial, sagittal, and coronal views are used. It is not clinically essential to pinpoint the exact pulmonary artery segment involved.
Pulmonary perfusion scanning makes the most assumptions about the particular anatomic segment involved, because actual anatomy is not visualized, but inferred by location (i.e., where, peripherally, the perfusion defect is seen and what normally perfuses that location).
Clinical parameters have some ability to predict fatality risk from PE in patients with deep venous thrombosis ( Table 28-1 ).
| CASE REPORT | % RISK OF FATAL PULMONARY EMBOLISM ( n = 15,520) |
|---|---|
| Patient < 75 years, deep vein thrombosis | 0.23 |
| Patient < 75 years, nonmassive symptomatic pulmonary embolism | 1.24 |
| Patient > 75 years, nonmassive symptomatic pulmonary embolism | 3.42 |
| Patient > 75 years, nonmassive symptomatic pulmonary embolism, immobilization > 4 days for neurologic disease | 9.81 |
| Patient > 75 years, massive pulmonary embolism, immobilization > 4 days for neurologic disease | 24.70 |
Screening and Supportive Diagnostic Testing for Pulmonary Embolism
Venous Doppler Examination
Venous Doppler examination is widely available and often used to assess suspected venous thromboembolic disease. Its use is widely accepted for the evaluation of symptomatic deep venous thrombosis (DVT) (venous Doppler is insensitive for asymptomatic DVT), but controversial for screening for PE. Older contrast venography studies established that one fifth of cases of PE had negative venograms. The number is considerably higher with venous Doppler examinations—one third to one half. The greatest protocol variation is inclusion or exclusion of the distal venous circulation. The role and utility of venous Doppler scanning are variable, depending on the center where it is being done. Its contribution to the assessment of PE is limited. A strategy of initial whole-leg scanning with color Doppler assessment versus two-point (i.e., superior femoral artery [SFA] and popliteal artery) serial scans (1 week apart) in patients with positive D-dimers appears to provide equivalent information. Lack of familiarity with venous anatomic variants such as bifid superficial femoral veins or bifid popliteal veins, and scanning only the readily imaged portion of the popliteal vein, diminish the utility of Doppler assessment as performed in such an abbreviated fashion.
D-dimer Blood Test
The D-dimer blood test for degraded cross-linked fibrin is widely available. There is assay variation (e.g., enzyme-linked immunosorbent assay [ELISA], latex, whole agglutination), and threshold variation. The usual threshold is 500 μg/L -1 . In an emergency department scenario, its sensitivity for thromboembolic disease is very high (97%) and the negative predictive value is excellent (99.7%). Specificity, however, is poor, particularly among the in-patient ranks, because many pulmonary illnesses, including pneumonia and sepsis, and the postoperative state, as well as healthy pregnancy, are associated with elevated D-dimers. Although a negative D-dimer test—in patients either with or without prior venous thromboembolism—has excellent negative predictive value (100%), a negative D-dimer test is uncommon in patients with prior venous thromboembolism.
The differential diagnosis of a positive D-dimer blood test includes:
- □
Trauma
- □
Inflammation
- □
Liver disease
- □
Malignancy
- □
Advanced age
- □
Elevated rheumatoid factor
- □
Postoperative state
- □
Pneumonia
- □
Sepsis
- □
Aortic dissection
- □
Myocardial infarction
- □
Stroke
- □
Atrial fibrillation
- □
Acute limb ischemia
- □
Intracardiac thrombus
- □
Disseminated intravascular coagulation
- □
Preeclampsia and eclampsia
- □
Use of thrombolytic agents
- □
Sickle cell disease
- □
Renal disease
- □
Nephrotic syndrome (e,g., renal vein thrombosis )
- □
Oral contraceptive use
- □
Ovarian cyst rupture
- □
Venous malformations
Diagnostic Testing for Pulmonary Embolism
Contrast Catheter-Based Pulmonary Angiography
Contrast catheter-based pulmonary angiography formerly was the “gold standard” for assessment of PE, but it is invasive (which is relevant with respect to anticoagulation and thrombolysis risks) and has imperfect interobserver agreement: only 45% to 66% for subsegmental emboli. A negative pulmonary angiogram, in the context of a suspected pulmonary embolus, using super-selective magnification, has an excellent associated 6-month death rate from thromboembolism or recurrent embolism rate of less than 1% (0/180). Vessel superimposition, as occurs with severe lung volume loss, renders image analysis difficult. Catheter-based pulmonary angiography may be used to enable catheter-directed thrombolysis. Motion and other technical problems for CT angiography are less of a problem for catheter-based angiography; hence pulmonary angiography may be used to evaluate cases of suspected PE with poor-quality CT pulmonary angiograms.
Gadolinium Contrast-Enhanced MR Angiography
Gadolinium contrast-enhanced MRA lacks the spatial resolution to demonstrate peripheral emboli, is poorly suited to the evaluation of ill patients, and lacks general availability. It cannot yet be considered a standard test, but the technology is developing. A role may potentially emerge for it in the assessment of suspected PE in pregnancy with nondiagnostic CT PE studies.
Nuclear Scintigraphy
Nuclear scintigraphy studies, although they potentially offer excellent negative predictive value, now are less likely to be used because they yield a very high proportion of nondiagnostic indeterminate studies (73% in the Prospective Investigation of Pulmonary Embolism Diagnosis [PIOPED] Study), they are less suitable to in-patient and postoperative cases, and they offer limited availability and lower interobserver correlation (when compared with CTA). Furthermore, in the PIOPED study, 12% of high-probability scans had normal pulmonary angiograms. The utility of ventilation/perfusion testing, especially for postoperative patients, critically ill in-patients, and patients with substantial lung or cardiac disease, added impetus to development and validation of tests short of pulmonary angiography. Although safer than CT scanning, over half of test results establish intermediate probability, and there is a 10% false-positive rate with high-probability scans and a 10% false-negative rate with low-probability scans. Still, a clear unmatched segmental defect is a useful finding.
CT Pulmonary Angiography
CT pulmonary angiography became the de facto test of choice for diagnosis of PE because of its ability to visualize thrombi directly, the widespread and generally 24-hour availability of CT scanning, the suitability and track record of CT scanning in general to evaluate critically ill patients, and its robustness in its diagnostic ability in identifying other major intrathoracic pathologies that are present in a high (often >50%) proportion of patients suspected of having PE. Interobserver variability is far better by CT (κ = 0.72) than by nuclear scintigraphy (κ = 0.22). Sensitivity for detection of emboli in the main arteries and segmental arteries is excellent, even by older-generation scanners. Older-generation scanners (single-slice CT [SSCT]) reported sensitivities of only 70% (compared with angiography), although that was for the detection of subsegmental emboli. Current cardiovascular CT systems have greater spatial resolution, have superior isotropic resolution, are less affected by partial volume averaging effects, and appear to have superior sensitivity. Maximum intensity projection (MIP) reformatting is poorly suited to the evaluation of PE because of its tendency to maximize high attenuation, thus underrepresenting small (low-attenuation) emboli.
Approximately one third (30% ) of emboli are seen within vessels smaller than lobar and segmental pulmonary arteries (“subsegmental arteries”) on pulmonary angiography; only about one third of these were detectable by SSCT, and the interobserver agreement of SSCT for small peripheral emboli was very poor. The clinical significance of isolated small peripheral pulmonary emboli is uncertain, and the issue of treatment of small isolated peripheral emboli is controversial. The clinical significance of widespread small peripheral emboli is less controversial. Because of the advent of and advances in multidirectional CT (MDCT), the small, isolated subsegmental pulmonary embolus is now a more common finding, but it proves to be a vexation as well. The lesion is beneath the resolution of predictive value of scintigraphy; few cases will undergo correlative pulmonary angiography, which has poor interobserver agreement for subsegmental emboli; and, furthermore, the medical literature and community are unsure of the correct management of these emboli.
Earlier, CT scanning showed promise, but clearly had clinically imperfect sensitivity for PE, and concern arose about the safety of withholding anticoagulation on the basis of CT pulmonary angiography, given its imperfect sensitivity. The sensitivity of MDCT for detection of pulmonary emboli is evolving, and until well tested with contemporary 64-slice detector systems and newer, wide-detector 256- and 320-slice systems, dual energy systems with lung perfusion (blood volume), and new non-gadolinium detector systems, remains unknown.
MDCT is more reliable when assessing for more central rather than far peripheral emboli.
Although the mortality of untreated PE is related to how proximal the untreated PE is, small peripheral pulmonary emboli may have considerable hemodynamic consequences in critically ill patients.
CT Pulmonary Angiography Image Data Set Review
- □
The CT scan data should be reviewed on axial images, not MIPs, because the MIP modality underrepresents low-attenuation structures such as emboli.
- □
Sagittal and coronary views may be helpful.
- □
IV contrast is obviously a prerequisite for a PE protocol study, but sometimes it is a relative contraindication due, for example, to advanced renal insufficiency.
Ancillary Findings on CT Pulmonary Angiography
- □
Right ventricular enlargement, in which the diameter of the right ventricle is greater than that of the left ventricle:
- •
On CT, the axial four-chamber view is 83% sensitive (but only 49% specific) for major adverse clinical events and has a relative risk of 4.2 [1.06–15.19]. See Figure 28-2 .

Figure 28-2
Dilatation of and reflux of contrast material into the azygous vein ( A, arrow ), as well as reflux of contrast material into the hepatic veins ( B, arrows ) in a 64-year-old man with acute pulmonary embolism. Both are nonspecific signs of right ventricular dysfunction (RVD). C, “Septal bowing” of the interventricular septum toward the left ventricle ( green lines ) in a 55-year-old woman with acute central pulmonary embolism and echocardiographically confirmed severe RVD.
(Reprinted with permission from Henzler T, Barraza JM Jr, Nance JW Jr, et al. CT imaging of acute pulmonary embolism. J Cardiovasc Comput Tomogr . 2011;51:3-11.)
- •
- □
Reflux of dye into the inferior vena cava (IVC):
- •
Reflux of dye (injected via an arm vein) into the IVC is seen in pulmonary hypertension, right ventricular systolic dysfunction, right ventricular diastolic dysfunction (pericardial constriction), and tricuspid regurgitation.
- •
The length of the column of dye reflux down the IVC correlates ( r = 0.84) with echocardiography-derived estimates of pulmonary hypertension.
- •
CT Criteria for the Diagnosis of Pulmonary Embolism
CT criteria for the diagnosis of PE include the following:
- □
Partial intraluminal filling defect
- □
Complete intraluminal filling defect
- □
Abrupt cessation of blood flow
- □
“Railway tracks”
- □
Mural defects
The following findings are not considered criteria of PE:
- □
Abrupt tapering of a pulmonary artery
- □
Decrease in blood flow
- □
Indirect signs such as a wedge-shaped pleural-based parenchymal shadow corresponding to pulmonary infarction
Underlying respiratory disease does not appear to reduce the negative predictive value of MDCT.
Thin collimation and breath-holding improve image quality and negative predictive value. Inability to breath-hold is not an exclusion criterion for CT assessment. Images are generally assessed with lung windows (window width 1500 HU; window center 500 HU) and mediastinal windows (window width 400 HU; window center 400 HU) as well. A high degree of vascular opacification (>200 HU) is desirable. The figures in this chapter depict a number of variants and complications:
- □
PE detected on CT ( Fig. 28-3 )

Figure 28-3
Axial, coronal, and sagittal maximum intensity projection reformations of a 34-year-old man with acute central pulmonary embolism and multiple segmental and subsegmental clots. Thin-section CT pulmonary angiography allows image reformations that improve the detection of segmental and subsegmental clots.
(Reprinted with permission from Henzler T, Barraza JM Jr, Nance JW Jr, et al. CT imaging of acute pulmonary embolism. J Cardiovasc Comput Tomogr . 2011;51:3-11.)
- □
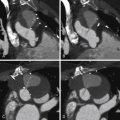
Cavitation of pleural-based infarcts ( Fig. 28-4 )

Figure 28-4
Pulmonary embolism and pulmonary infarction. A and B, Contrast-enhanced CT scans of a patient with a pulmonary embolism and a pleural-based wedge-shaped infarction of the left lower lobe. There is a pleural effusion as well. C and D, Contrast-enhanced CT scans of a patient with a pulmonary embolism and a cavitated pleural-based wedge-shaped infarction of the left lower lobe. There is a pleural effusion as well. E and F, Pulmonary embolism and infarction. Contrast-enhanced CT scans of a patient with a pulmonary embolism and a cavitated pleural-based wedge-shaped infarction of the left upper lobe.
- □
Submassive and massive PEs ( Figs. 28-5 and 28-6 )

Figure 28-5
Chest radiographs and contrast-enhanced CT scan views of a patient with pulmonary hypertension from chronic recurrent pulmonary embolism. The right ventricle and the main and central pulmonary arteries are dilated. A large burden of clot is present centrally within the pulmonary arteries. The peripheral arteries are diminished in size and appear few in number.

Figure 28-6
CT pulmonary angiography: note the high concentration of contrast in the right ventricle. There are bilateral pulmonary emboli, and the right heart is clearly dilated due to right heart strain from pulmonary vascular obstruction.
- □
Cases with pleural-based infarction (Hampton humps; Fig. 28-7 )

Figure 28-7
Contrast-enhanced CT scans of a patient with pulmonary embolism and infarct of the left upper lobe. The pulmonary infarction, with its characteristic pleural-based triangular or wedge shape, is seen more easily in the lung window. The aorta is mildly dilated and heavily atherosclerotic.
- □
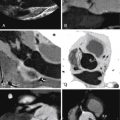
Emboli in-transit: usually, emboli stick into a patent foramen ovale (PFO) due to right heart failure and an underlying PFO that “caught” an otherwise pulmonary embolism as it was directed by hydrostatic pressures to traverse a PFO when the embolism arrived in the right atrium during systole ( Figs. 28-8 and 28-9 )

Figure 28-8
Comprehensive cardiothoracic CT examination. A, Axial image at the level of the pulmonary artery bifurcation shows bilateral pulmonary embolism ( arrows ). B, The four-chamber image shows right ventricular enlargement consistent with right ventricular strain and leftward bowing of the interatrial septum ( arrow ) consistent with elevated right-sided pressures. C, The modified four-chamber image shows thrombus in transit ( arrow ) straddling a patent foramen ovale. D, Parasagittal image at the level of the atria also shows thrombus in transit ( arrow ). E and F, Intraoperative findings. E, Thrombus was seen extending through the patent foramen ovale ( arrow ) at the time of surgery. F, Intracardiac thrombus after removal. LA, left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle.
(Reprinted with permission from Syed IS, Motiei A, Connolly HM, Dearani JA. Pulmonary embolism, right ventricular strain, and intracardiac thrombus-in-transit: evaluation using comprehensive cardiothoracic computed tomography. J Cardiovasc Comput Tomogr . 2009;33:184-186.)

Figure 28-9
A composite image in a young woman after a high-speed motor vehicle accident. The patient incurred an acute aortic injury that is best seen on the sagittal reconstruction through the chest ( B ). An underlying left posterior pulmonary contusion is also noted ( A and B ). A and B, CT images also demonstrate a small soft tissue density affixed to the interatrial septum, residing within the left atrium. C, This was confirmed on TEE as an embolism in transit, across the foramen ovale.
- □
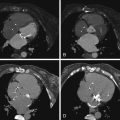
Segmental oligemia (Westermark sign; Fig. 28-10 )

Figure 28-10
CT angiography of a 78-year-old woman with chest pain and acute shortness of breath. A, The anteroposterior chest radiograph demonstrates moderate enlargement of the cardiac silhouette. There is also appreciable asymmetry in pulmonary arterial vascular markings within the lungs, with the right lung demonstrating much less peripheral pulmonary arterial vasculature. This finding is known as a Westermark sign. B, Lung reconstruction from a CT pulmonary embolism (PE) study also demonstrates asymmetry in pulmonary arterial vasculature between the right lung (decreased on the right). The remaining CT images from the same CT PE study demonstrate a large burden of thrombus within the right pulmonary arterial tree, and no definite left-sided pulmonary embolism. F, Secondary signs of elevated pulmonary artery pressure are present, with an enlarged right ventricle and right atrium.
Although the clinical risk to patients with suspected PE and normal pulmonary angiography is small, it is measurable, and higher than that of the general hospitalized patient population, which suggests that a more comprehensive assessment, such as inclusion of lower limb Doppler ultrasound studies, may be useful.
The following issues may be relevant in the assessment of patients with suspected PE:
- □
Pulmonary emboli tend to fragment with time, and the residua embolize more distally. Thus, the detection rate directly relates to the promptness of testing, and detection may be forfeited if unduly delayed.
- □
Cost
- □
Invasiveness of testing and the tolerability of complications
- □
Patient’s ability to tolerate contrast dye
- □
Patient’s ability to tolerate transport to either the CT scanner or the angiography suite
- □
Bleeding rates from heparin (per day):
- •
Fatal: 0.05%
- •
Major: 0.8%
- •
Minor: 2%
- •
It appears increasingly less likely that CVCT can ever be formally compared in a large trial to pulmonary angiography, which has been the traditional gold standard for PE diagnosis and now is the true standard.
Algorithms for Assessment of Pulmonary Embolism
The optimal use of CT to evaluate cases of suspected PE is still not entirely clear. The question is really in what algorithm (i.e., combination and sequence with other testing) CT scanning should be employed. The tests most commonly combined with CT scanning are D-dimer assay and venous leg Doppler examination. Increasingly, emphasis is placed on detailed clinical risk assessment ( Table 28-2 ) in combination with testing. D-dimer assays do not all appear comparable, and there is a tendency to shorten algorithms (i.e., omit lower limb Doppler ultrasound), although this may simply reflect more of a drive toward expediency, away from comprehensiveness.
| CLINICAL FEATURE | SCORE |
|---|---|
| Clinical signs and symptoms of DVT (objectively measured leg swelling and palpation in the deep vein system) | 3.0 |
| Heart rate >100 beats/min | 1.5 |
| Immobilization for ≥3 consecutive days (bed rest except to go to bathroom) or surgery in previous 4 weeks | 1.5 |
| Previous objectively diagnosed pulmonary embolism or DVT | 1.5 |
| Hemoptysis | 1.0 |
| Cancer (with treatment within past 6 mo or palliative treatment) | 1.0 |
| Pulmonary embolism likely or more likely than alternative diagnoses (on the basis of history, physical examination, chest radiography, ECG, and blood tests) | 3.0 |
∗ Scoring method indicates high probability if score is 3 or more; moderate if score is 1 or 2; and low if score is 0 or less.
Bayesian theory holds true—that is, that the post-CTA probability of PE is significantly skewed by the pretest probability, which can be approximated by clinical assessment ( Table 28-3 ).
| VARIABLE | HIGH CLINICAL PROBABILITY | INTERMEDIATE CLINICAL PROBABILITY | LOW CLINICAL PROBABILITY | |||
|---|---|---|---|---|---|---|
| NO./TOTAL NO. | VALUE (95% CI) | NO./TOTAL NO. | VALUE (95% CI) | NO./TOTAL NO. | VALUE(95% CI) | |
| Positive predictive value of CTA | 22/23 | 96 (78–99) | 93/101 | 92 (84–96) | 22/38 | 58 (40–73) |
| Positive predictive value of CTA or CTV | 27/28 | 96 (81–99) | 100/111 | 90 (82–94) | 24/42 | 57 (40–72) |
| Negative predictive value of CTA | 9/15 | 60 (32–83) | 121/136 | 89 (82–93) | 158/164 † | 96 (92–98) |
| Negative predictive value of both CTA and CTV | 9/11 | 82 (48–97) | 114/124 | 92 (85–96) | 146/151 † | 97 (92–98) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree