Ashish Aneja, MD
OVERVIEW OF THE ROLE OF CARDIAC MRI IN PULMONARY HYPERTENSION
Pulmonary Hypertension (PH) is defined by a mean pulmonary arterial pressure (mPAP) over 25 mm Hg at rest and over 30 mm Hg with exercise. A resting pulmonary capillary wedge pressure (PCWP) of 15 mm Hg is used to differentiate between pre- and postcapillary PH, with the latter suggested by a PCWP exceeding this threshold.1
The most widely accepted modality to screen for PH is echocardiography (ECHO); it has excellent Doppler techniques that estimate the pulmonary artery pressures.2 Using the Bernoulli equation to estimate right ventricular systolic pressure (RVSP), a tricuspid insufficiency peak gradient (TIPG) ≥ 30 mm Hg has been used as an arbitrary criterion for noninvasive diagnosis of PH.1 However, ECHO may be limited due to the patient’s body habitus, which will limit the quality of the data. Even with optimal images the entire right heart is not visualized because of the inherent limitations imposed by the acoustic window. A delay in recognition of pulmonary arterial hypertension (PAH) has been identified.3 The current gold standard for the assessment of PH is right heart catheterization.4,5 This is an invasive procedure that has inherent risks such as bleeding, infection, vascular trauma, pneumothorax, and potentially death. However, this procedure carries critical information in regards to etiology, therapeutic options, and prognosis.
In contrast, cardiac magnetic resonance (CMR) enjoys the unique advantages of being noninvasive yet providing excellent resolution utilizing nontoxic (in those without renal dysfunction or other risk factors for nephrogenic systemic fibrosis) contrast and the ability to image in any three-dimensional (3D) plane. However, a lack of widespread availability, historically long scanning times, claustrophobia, necessity of breath-holding, and relative contraindications in individuals with pacemakers, defibrillators, and aneurysm clips may impose some limitations. Due to the unique advantages, CMR is widely considered the gold standard for evaluation of the complex right ventricular (RV) anatomy and function, making it ideally suited for PH assessment because of disproportionate RV involvement.6,7 Accurate measurements of RV volume, mass, myocardial contractility, and pulmonary arterial flow, in addition to use of delayed enhancement (DE) imaging (Figure 12-1) make CMR a valuable tool in diagnosis, prognostication, and treatment monitoring.8–12 The typical CMR protocol in PH includes cine imaging in standard planes, first-pass imaging to assess for intracardiac shunts, flow quantitation across the aortic root and main pulmonary artery (MPA), and late post-gadolinium imaging. Magnetic resonanace angiography (MRA) may be included to assess for thromboembolic disease and vascular anomalies.
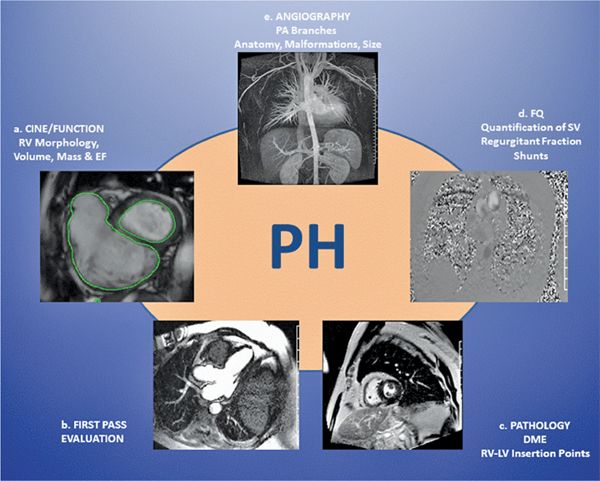
FIGURE 12-1 Integrated techniques for the various aspects of pulmonary hypertension (PH) make cardiovascular magnetic resonance (CMR) a useful modality in PH evaluation.
CMR sequences that make blood appear dark (black-blood half-Fourier acquisition single-shot turbo spin-echo imaging, also known as dark-blood HASTE), or bright (such as steady-state free precession or SSFP imaging) allow for excellent anatomical definition. SSFP imaging is the currently favored technique for cine CMR images utilizing 5- to 18-second breath-hold, depending on heart rate (HR). Contiguous short-axis SSFP cines that are typically 6 to 8 mm in thickness yield accurate RV and left ventricular (LV) mass, volume, and ejection fractions (EF). From a prognostic standpoint, CMR-derived RV end-diastolic volume (RVEDV) index of > 84 mL/m2 and an LV end-diastolic volume (LVEDV) index of < 40 mL/m2 indicate reduced 1-year survival.13 Recently, CMR-derived RVEF was determined to be an independent predictor of clinical worsening in a study of 58 PH patients (of diverse etiology) referred for CMR who also underwent right heart catheterization (RHC), functional capacity measurement, and biomarker assess-ment.14 In 37 PH patients undergoing CMR and RHC, an abnormal interventricular septum curvature ratio assessed with CMR revealed a direct correlation between a systolic PAP > 67 mm Hg and altered LV curvature (r = 0.77).15 Similarly, in 61 patients undergoing CMR and RHC the same day, rate of LV pressure rise correlated well with LV curvature ratio (r = 0.85). Moderate agreement between cardiac MR- and RHC-derived pulmonary artery systolic pressure (PASP) was observed.16 In patients with chronic thromboembolic pulmonary hypertension (CTEPH), RVEF estimated by CMR correlated well with pulmonary vascular resistance (PVR) (r = 0.6) and mean pulmonary artery pressure (mPAP) (0.7 mm Hg). In the postoperative phase, recovery of mPAP correlated well with RVEF (r = 0.8).17 Additional CMR assessed parameters include RV/LV ventricular mass index (VMI) > 0.6, which correlated well with mPAP (r = 0.8) in one study comparing PH patients and normal controls.18 A more recent larger study confirmed these findings and demonstrated reduced 3-year survival in patients with a VMI exceeding 0.75,19 but the relationship between VMI and PAP has not been consistently reproduced by all groups.20 When RV volumes and mass were compared with traditional measures such as New York Heart Association (NYHA) class, 6-minute walk distance (6MWD), and RHC derived values, an RVEDV > 84 mL/m2 was found to be associated with mortality.13 Perhaps more importantly, the study demonstrated the poor prognostic value of a low-LV ventricular mass index, presumably due to poor RV function and interventricular septal encroachment upon the LV.13
CMR has been used in a small study to guide invasive RHC, coupled with MRI-assessed PA blood flow and phasic RV volumes, detailed information about RV overload, pump function, myocardial contractility, and ventriculo-arterial coupling following a porcine validation study.12 The same group used CMR-guided RHC to assess changes in PVR in idiopathic PH patients.21 These single-center experiences have paved the way for further technological developments in this field, with the distinct advantages of no radiation and nephrotoxic contrast.
Assessment of RV diastolic function by CMR has been facilitated by measuring isovolumic relaxation time (IVRT)—the interval between closure of the pulmonary and opening of the tricuspid valves. Not only does IVRT correlate well with RV mass and PV,22 but also improves in response to PH therapies such as sildenafil.23 Measurement of CMR-derived tricuspid inflow velocity and tricuspid annular velocity (E/A) ratio normalized to RV end-diastolic volume also yielded useful diagnostic information. Myocardial tagging, a noninvasive CMR technique that measures myocardial deformation and 3D motion, has demonstrated a significant interventricular asynchrony and a prolonged RV ejection time compared to the LV, which likely contributes to poor LV filling in PH patients.24
Late gadolinium enhancement (LGE) techniques in PH patients have demonstrated enhancement at the RV insertions points in the septum, extending to the interventricular septum in many cases. These abnormal areas are thought to result from mechanical stress (not proven) and frequently associated with septal flattening or bowing, reflecting high right-sided pressures.8,25 The extent of LGE abnormality has been inversely associated with RV functional measures.25
Velocity-encoded (VENC) phase-contrast (PC) CMR enables accurate measurements of flow velocity and volume. A product of the velocity and cross-sectional area is used to compute flow, stroke volume, and cardiac output. Studies have demonstrated important prognostic implications for stroke volume assessed by CMR. A 10-mL or greater increase in stroke volume during PH treatment is thought to represent significant improvement, making it an appealing endpoint for therapeutic trials.13,26,27 Studies have also demonstrated that PH patients have nonhomogeneous PA velocity, large amounts of retrograde flow, and reduced PA distensibility. PH patients also demonstrated shorter time-to-peak PA velocity and a steeper velocity increase gradient than normal subjects. In CTEPH patients, postoperative peak flow velocity was significantly improved compared to the preoperative state.28 CMR can also be used to derive additional flow measures such as the acceleration time (AT)—time from onset to the peak velocity of flow in the MPA, which in turn has been used to assess PA pressure and/or PVR.19
Pulmonary artery distensibility, mentioned briefly above, has recently received attention as a measure of PH, being lower than in normal subjects. In idiopathic PH patients, changes in PA distensbility mirror vasodilator responsiveness and mortality.29,30
MR pulmonary angiography has also been evaluated in CTEPH patients and is successfully able to image up to the level of the segmental arteries.31 In a retrospective analysis, contrast-enhanced (CE) pulmonary angiography had a high sensitivity and specificity for the diagnosis of lobar and segmental disease, but not subsegmental processes.32 Additionally, MR pulmonary angiography can demonstrate dilated PAs that act as collaterals to regions of reduced perfusion by pulmonary arteries, often noted in CTEPH patients. Time-resolved contrast pulmonary angiography has demonstrated prolonged pulmonary artery transit times in PH patients compared to normal subjects, which appears promising for use in assessing altered hemodynamics in these patients.33
OVERVIEW OF THE ROLE OF CT IN PULMONARY HYPERTENSION
The advantages of CT include a vivid delineation of pulmonary parenchymal disease, ready availability, short scan times, and limited breath-holds. Obvious disadvantages include exposure to ionizing radiation and nephrotoxic contrast, especially in patients who require multiple studies.
In patients with precapillary PH, CT demonstrates PA dilatation and pruning of the peripheral vasculature.34 In PH patients, CT angiography often demonstrates RV dilatation, hypertrophy, septal flattening/bowing, superior and inferior vena cava (SVC and IVC respectively) dilation with contrast regurgitation due to tricuspid regurgitation.35 Despite the established value of ventilation/perfusion (V/Q) scanning in patients with CTEPH, CT scanning can help differentiate other causes of V/Q mismatch, including pulmonary veno-occlusive disease, tumors and extrinsic PA compression, and vasculitides.36 In CTEPH patients, CT can variably demonstrate vessel occlusion, partial recanalization, and pulmonary infarcts. Like MR pulmonary angiography, CT pulmonary angiography can effectively define bronchial artery collaterals, which can either be nonspecific or represent compensatory perfusion.37
In post-capillary PH patients, CT typically demonstrates findings of interstitial pulmonary edema. When postcapillary PH is due to LV dysfunction, reduced LV function and LV/LA enlargement/hypertrophy can be demonstrated. Rarer causes of PH including pulmonary veno-occlusive disease (PVOD) and pulmonary capillary hemangiomatosis, which can respond adversely to pulmonary vasodilator therapy, can display characteristic CT findings including dependent centrilobular ground-glass opacities, mediastinal lymph node enlargement, subpleural septal lines, and pleural effusions.38
The main pulmonary artery diameter (MPAD) exceeding 29 mm by CTA has a high sensitivity and specificity in PH patients39 that has been variably correlated with invasive PA pressures. More recently, a PA to aorta ratio of > 1 has been noted to be a better identifier of PH than PA diameter alone,40 since it may reflect elevated PA pressures and reduced systemic cardiac output.41 The ratio of segmental PA diameter to the outer diameter of the adjacent bronchus (ratio > 1.25) in 3 out of 4 pulmonary segments has been shown to not only have a high sensitivity and specificity for the diagnosis of PH, but also the severity of PH.42
The subsequent sections of this chapter utilize a case-based approach demonstrating the utility of CMR and CT in the diagnosis and follow-up of PH patients. The World Health Organization (WHO) classification of pulmonary hypertension is listed in Table 12-1 at the end of this chapter.
CASE STUDIES
WHO CLASS I: PULMONARY ARTERIAL HYPERTENSION
CASE 1 Idiopathic Pulmonary Arterial Hypertension (IPAH)
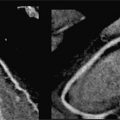
A 41-year-old patient with known severe idiopathic PH, estimated RVSP of 110 mm Hg by a recent ECHO, NYHA class II on maximal medical therapy, underwent a CMR examination that showed RV enlargement (RVE), RV hypertrophy (RVH), and a diminished RV ejection fraction (EF) of 35%. The LV cavity appeared small with pronounced systolic flattening of the interventricular septum, consistent with RV pressure overload. The PA was enlarged. There was also hyper-enhancement at the RV-LV insertion sites by LGE. This CMR pattern is characteristic of advanced pulmonary hypertension (Figure 12-2, and Videos 12-1 and 12-2).
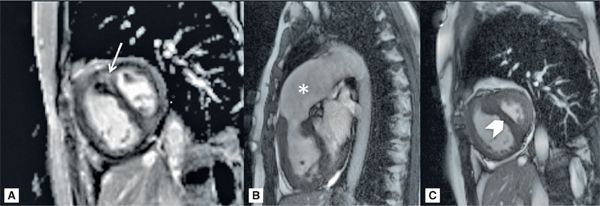
FIGURE 12-2 CMR in a patient with severe PH. (A) A short-axis late gadolinium enhancement (LGE) image in a patient with severe pulmonary hypertension shows marked hyperenhancement at the septal insertion sites extending into the interventricular septum (arrow). (B) Frame from right ventricular outflow plane cine demonstrates RVH and a dilated PA (asterisk). (C) Short-axis cine systolic image shows a significantly larger RV vs LV with flattening of the interventricular septum (chevron).
CASE 2 Pulmonary Artery Enlargement in Pulmonary Hypertension
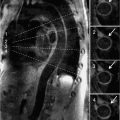
A 61-year-old patient with history of severe idiopathic PH had the following PAP by RHC: Systolic/Diastolic/Mean (S/D/M) 111/40/68 mm Hg. CMR examination was ordered to assess RV function and revealed moderately severe pulmonary insufficiency (PI) with markedly dilated proximal pulmonary vasculature (Figure 12-3 and Video 12-3). The RV appeared severely dilated with severe systolic dysfunction, RVEF 23%. In addition, there was a significantly prolonged pulmonary to aorta transit time of 9 seconds (Figure 12-4).
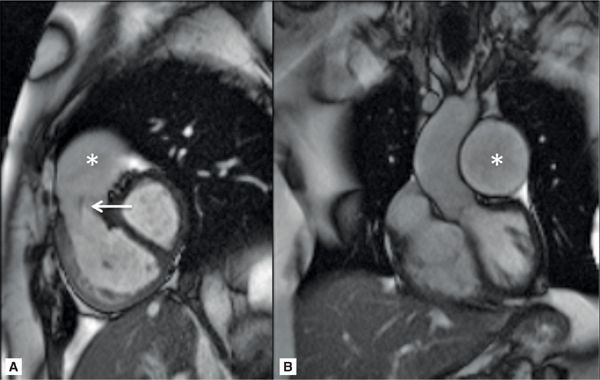
FIGURE 12-3 CMR imaging in a patient with severe PH. (A) RV-outflow cine image reveals a dilated RV, a jet of pulmonary regurgitation (PR) (arrow), and a dilated PA (asterisk). (B) A left ventricular outflow tract (LVOt) cine image showing a markedly enlarged left PA (asterisk).

FIGURE 12-4 Individual frames from a first-pass shunt evaluation scan, where 1 frame is acquired each second. The initial frame with arrival of contrast in the right heart is shown as time 0, and the frame showing initial appearance of contrast in the LA (arrow) is 9 seconds later. This indicates a pulmonary to aorta transit time of 9 seconds, which is significantly prolonged in this patient due to severe PH.
CASE 3 Connective Tissue Disease
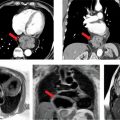
A 58-year-old patient with history of PH secondary to CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, and telangiectasia) had a recent RHC showing PAP: S/D/M of 65/34/46 mm Hg and wedge pressure of 11 mm Hg. CMR (Figure 12-5 and Video 12-4) revealed paradoxical interventricular septal motion with dilated MPA, RV enlargement, and global systolic dysfunction. A focal mid-wall scar near the inferior RV insertion site was seen.
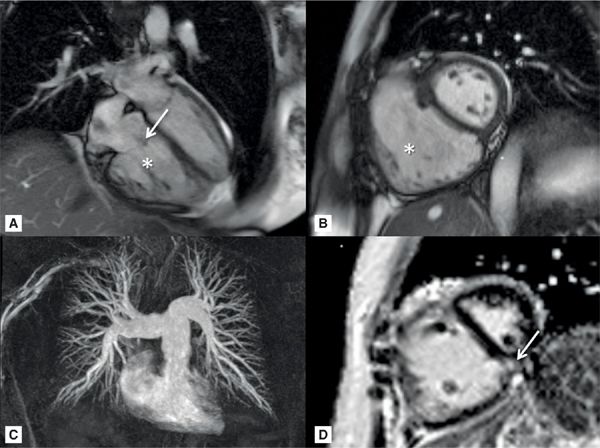
FIGURE 12-5 CMR acquisitions in a patient with severe PH due to CREST include HLA (A) and short-axis (B) SSFP cine frames showing RVE (asterisk) and TR (arrow). Maximal Intensity Projection (MIP) of a pulmonary angiogram shows dilated central and branch PAs (C). Late gadolinium enhancement (LGE) short-axis reveals prominent mid-wall enhancement at the inferior septal insertion site (D, arrow)—a typical pattern seen in severe PH.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree