Systemic diseases often manifest with cutaneous findings. Many pediatric conditions with prominent skin findings also have significant pulmonary morbidity. These conditions include inherited multisystem genetic disorders, such as yellow nail syndrome, neurofibromatosis type 1 (NF1), tuberous sclerosis complex (TSC), hereditary hemorrhagic telangiectasia (HHT), Klippel-Trénaunay-Weber syndrome, and Ehlers-Danlos syndrome (EDS), and reactive processes, such as xanthoma disseminatum and mastocytosis. This chapter discusses the common presentations and pulmonary manifestations of these disorders.
Yellow Nail Syndrome
Yellow nail syndrome was described first in 1964 in a group of 13 patients with lymphedema and distinctive nail findings. Yellow nail syndrome is mostly a disease of early middle age, affecting women more commonly than men by 1.6:1 ; however, neonatal chylothorax has been described in association with yellow nail syndrome. It also has been reported in a male infant born with congenital lymphedema, who developed bilateral pleural effusions and a pericardial effusion at 6 months of age. The classic clinical presentation includes yellowish nail discoloration and dystrophy (89%), lymphedema (80%), and pleuropulmonary disease (63%). Two of the three clinical manifestations are required for the diagnosis of yellow nail syndrome. The cause of this syndrome remains unknown, although a few cases apparently followed episodes of pneumonia. Many cases have been attributed to congenital lymphatic hypoplasia, similar to that occurring in primary lymphedema. Yellow nail syndrome has been associated with numerous malignancies and immunodeficiencies.
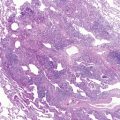
Yellow nail syndrome is characterized by thickened, yellowish gray, slow-growing nails that are excessively curved along both axes. Nail changes are insidious in onset, are bilateral, and affect hands and feet ( Fig. 12-1 ). Nail changes may occur before or after other physical findings. Cuticles and lunula may be absent, and fragility and separation from the nail plate (onycholysis) may be seen. These findings may easily be confused with onychomycosis. Nail cultures are negative, and systemic antifungal therapy is unhelpful. Histopathologic changes in the nail matrix and bed show dense fibrous tissue, which replaces subungual stroma with numerous ectatic, endothelium-lined vessels that are similar to the pleural abnormalities found in yellow nail syndrome.

Primary lymphedema typically involves the upper and lower extremities. The lymphedema is believed to be secondary to congenital atresia or hypoplasia of the lymphatics. Lymphoscintigraphy is diagnostic.
The pleuropulmonary manifestations include pleural effusions and bronchiectasis. Pleural effusion may precede the onset of nail changes by several years. Pleural effusions range from small, unilateral, and asymptomatic to large, bilateral, and recurrent ( Fig. 12-2 ). The fluid can be an exudate or transudate. Empyema has been reported as a complication of yellow nail syndrome. The pleural effusion may be due to defective lymphatic drainage, rather than excess ce:anchor id=”me:f0010″ role=”moved-element”/>production of pleural fluid. Electron microscopy has revealed the presence of dilated lymphatic capillaries in the visceral pleura, suggesting an obstruction to the lymph drainage. Yellow nail syndrome associated with bilateral cystic lung disease was reported in a 4-year-old girl, which suggests that normal lymphatic drainage is essential for normal lung development.


There also is an increased incidence of sinusitis, bronchiectasis, and lower respiratory tract infections in patients with yellow nail syndrome, possibly related to an inherent immunodeficiency. Bronchiectasis affects mostly the upper lobes, and the cause is unknown. High-resolution computed tomography (CT) of the chest is diagnostic.
Other features that have been described in yellow nail syndrome include Raynaud phenomenon, keratosis obturans involving the external ear, excess cerumen, nephrotic syndrome, pericardial effusions, chylous ascites, intestinal lymphangiectasia, and selective antibody deficiency. Several cases of yellow nail syndrome in association with cancer of the breast, lung, and larynx have been reported. The nail changes are related to lymphatic obstruction, which is caused by the underlying malignancy.
Large, recurrent pleural effusions may require repeated thoracentesis, pleuroperitoneal shunting, chemical or surgical pleurodesis, or pleurectomy. Chylous effusions are more difficult to treat and may require dietary restriction of fat and supplements of medium-chain triglycerides. Treatment of the pulmonary disease or malignancy has resulted in resolution of nail changes.
Neurofibromatosis Type 1
NF1, also called von Recklinghausen disease or peripheral NF, is a common autosomal dominant neurocutaneous disorder. Virtually every organ system may be affected. These protean features may be present at birth or in early childhood, but complications are generally delayed for years.
NF1 occurs in 1 in 3000 individuals; there is no ethnic or gender predilection. NF1 has a high spontaneous mutation rate (50%) and variable expression. The gene responsible for NF1 is located on chromosome 17. The gene product, neurofibromin, subserves a tumor suppressor function, and loss of heterozygosity results in the hamartomatous multiorgan growth that accounts for the clinical heterogeneity seen in NF1.
Café au lait macules are the hallmark cutaneous finding in NF1 and are present in almost 100% of patients ( Fig. 12-3 ). Other cutaneous features may manifest slightly later in infancy or childhood, including axillary (Crowe sign) or inguinal freckling, or both, and neurofibromas. Rubbery discrete neurofibromas do not tend to occur until adolescence or later, although plexiform neurofibromas are generally present at birth ( Fig. 12-4 ). Plexiform neurofibromas grow indolently and have a distinctive “bag of worms” texture, and often have overlying hyperpigmentation and hypertrichosis. Deeper plexiform neurofibromas may cause asymmetry or localized gigantism.


Noncutaneous features include asymptomatic iris hamartomas (Lisch nodules). These nodules are present in 95% of NF1 patients by 10 years of age. Optic gliomas occur in 15%, but most are asymptomatic and nonprogressive. Skeletal features of NF1 include scoliosis, long bone dysplasia, nonossifying cyst formation, and short stature. A distinctive tibial anteromedial bowing manifests often in the first year of life, with medullary sclerosis and cortical thinning seen radiographically.
Neuropsychiatric disability is common, with 50% of NF1 patients having attention-deficit/hyperactivity disorder, learning disability, and verbal or nonverbal disability. Other features described in NF1 include hypertension secondary to renal artery stenosis or, less commonly, pheochromocytoma, macrocephaly, and increased malignancy risk for pilocytic astrocytomas, peripheral nerve sheath tumors, and leukemia.
Diagnosis is based on established clinical criteria ( Table 12-1 ). Gene testing also is available, and newer techniques have improved mutation detection rates to around 90%. Brain magnetic resonance imaging (MRI) may reveal unidentified bright objects, but these have unclear diagnostic and prognostic significance, and no established recommendation exists for routine brain imaging. Genetic evaluation of suspected patients and family members, ophthalmologic evaluation annually until 10 years of age for diagnostic screening in suspected patients, and annual dermatologic examinations with a Wood light are helpful.
| Six or more café au lait macules >5 mm in greatest diameter in prepubertal individuals and >15 mm in greatest diameter in postpubertal individuals |
| Axillary or inguinal freckling |
| Two or more iris Lisch nodules |
| Two or more neurofibromas or one plexiform neurofibroma |
| Distinctive osseous lesion such as sphenoid wing dysplasia or cortical thinning of long bones |
| Optic pathway gliomas |
| First-degree relative with neurofibromatosis type 1 whose diagnosis was based on aforementioned criteria |
Pulmonary involvement resulting from NF1 is uncommon and manifests later in childhood or early adulthood. Pulmonary manifestations include diffuse pulmonary fibrosis, bullous lung disease, endobronchial neurofibromas, and mediastinal masses. Pulmonary fibrosis is usually seen in the basal areas of the lungs, whereas bullous lesions occur predominantly in the apical areas. When mediastinal lesions are located in the posterior mediastinum, they may manifest as “dumbbell” tumors with intraspinal extension. MRI of the involved spinal area is helpful in assessing the anatomic relationships of such tumors. Primary pulmonary neurofibromas are rare. Affected patients typically present with dyspnea on exertion.
Chest radiographs and chest CT scans reveal reticulonodular infiltrates in the bases and bullous lesions in the apices. Histologically, alveolar septal fibrosis and alveolitis lead to a mixed obstructive and restrictive lung dysfunction. In 5% of patients with NF, the neurofibromas, regardless of their location, may undergo malignant degeneration and commonly metastasize to the lungs. “Scar” cancer of the lung can complicate long-standing NF1 pulmonary involvement.
Tuberous Sclerosis Complex
TSC is a neurocutaneous disorder characterized by intracranial abnormalities and distinctive skin markings. Historically, this disease was thought to consist of facial angiofibromas and disabling neurologic disorders, including epilepsy, mental retardation, and autism, but it is now acknowledged to affect many organ systems, including the eye, kidneys, heart, and lungs. Its incidence is approximately 1 in 6000.
TSC is a disorder of cellular differentiation and proliferation that is inherited as an autosomal dominant trait with variable expression and a high spontaneous mutation rate. Molecular genetic analysis has implicated two causal mutations in the genesis of TSC: TSC1 , on chromosome 9q34, encodes hamartin, and TSC2 , on chromosome 16p13, encodes tuberin.
The diagnostic criteria for TSC consist of major and minor features ( Table 12-2 ). Younger patients generally present with neurologic manifestations, whereas pulmonary manifestations are more common in later life. Cutaneous findings are age-related with 90% of affected patients having hypopigmented macules at birth or very early in infancy and childhood. The signature “ash-leaf macule” is most characteristic, but TSC-related hypopigmentation may be polygonal, confetti-like, or segmental ( Fig. 12-5 ). Three or more hypopigmented macules suggest TSC and should initiate a thorough family history and diagnostic work-up. Connective tissue nevi (Shagreen patch) typically occur on the trunk or face (fibrous forehead plaque) and may be seen at birth or early in life. Ungual fibromas (Koenen tumors) ( Fig. 12-6 ) and facial angiofibromas manifest later in childhood or early adolescence. Other organ systems involved include the eyes (“mulberry tumors” and retinal hamartomas), heart (rhabdomyomas), kidneys (cysts, angiomyolipomas), and lungs (lymphangiomyomas).
| Major features | Minor features |
|---|---|
|
|


Pulmonary involvement in TSC is uncommon, typically occurring in adolescent girls or women. Respiratory symptoms include chronic cough, progressive dyspnea, hemoptysis, and recurrent pneumothoraces. Pulmonary involvement is extremely rare in men or children. Unilateral lung cysts and a large pneumothorax were described in a 7-year-old boy with TSC. Recurrent acute respiratory distress syndrome secondary to TSC was reported in a 4-year-old boy.
Histopathologically, two patterns of lung involvement have been described: lymphangiomyomatosis, also called lymphangioleiomyomatosis, and micronodular pneumocyte hyperplasia/multifocal alveolar hyperplasia. Lymphangiomyomatosis occurs exclusively in women of childbearing age and is characterized by widespread proliferation of abnormal smooth muscle cells surrounding lymphatic and blood vessels and small airways.
Angiomyolipomas may produce generalized cystic or fibrotic changes in the lung and lead to spontaneous pneumothorax. Lymphatic obstruction may cause chylous effusion, and venous obstruction may lead to alveolar hemorrhage and hemoptysis. Bronchiolar obstruction results in air trapping and later lung cyst formation.
Radiographic evidence indicates that the incidence of lymphangiomyomatosis among women with TSC is 26% to 39%. Chest radiography may be normal or reveal diffuse interstitial lung disease or multifocal cysts. Thoracic CT may show variable thin-walled cysts scattered in all parts of the lungs, with normal-appearing lung tissue between cysts.
A thorough diagnostic evaluation should include a dermatologic examination with Wood light to assess subclinical pigmentary change and renal and cardiac ultrasound. Cardiac rhabdomyomas are congenital and may regress spontaneously by several years of age. Consequently, two-dimensional echocardiography in the newborn period is indicated if TSC is suspected. Renal angiomyolipomas and cysts are present in 15% of patients, and ultrasound or abdominal CT can detect even asymptomatic lesions. Although benign, renal lesions are the second most common cause of morbidity because of growth and parenchymal destruction resulting in hematuria, hypertension, and renal failure. An ophthalmologic examination may detect the pathognomonic retinal hamartomas, which occur in 76% of patients. A dental examination can identify enamel pits and craters in permanent teeth and gingival fibromas. Evaluation of the central nervous system is essential, including a thorough neurologic examination and contrast-enhanced CT or MRI.
Treatment for TSC is symptomatic. Many physical findings are generally stable and nonprogressive, such as the pigmentary and connective tissue changes in the skin. Other findings, such as cardiac rhabdomyomas, recede spontaneously.
Therapy for pulmonary TSC is similar to that for lymphangiomyomatosis, which some consider a forme fruste of TSC. Hormonal factors may play a role in the pathogenesis of lymphangiomyomatosis/TSC, which is consistent with typical presentation in premenopausal women, and are a potential therapeutic target. Medroxyprogesterone has been recommended when the disease becomes symptomatic or shows deterioration on pulmonary function tests. Oophorectomy, radiotherapy, or a combination of these has been beneficial. Other therapies that have shown some benefit include chemotherapeutic agents, such as cyclophosphamide. Tamoxifen has yielded mixed results to date, and corticosteroids are ineffective. Surgery has a role in pulmonary tuberous sclerosis to obtain a lung biopsy specimen for diagnosis, and to treat complications such as pneumothorax, to excise bullae, and to perform pleurodesis.
Because tumor cells from patients with TSC activate mammalian target of rapamycin (mTOR), mTOR inhibitor (sirolimus) has been identified as a potential therapeutic agent. The drug sirolimus suppresses mTOR signaling. The results of a clinical trial of sirolimus in patients with TSC and in patients with lymphangiomyomatosis revealed that lymphangiomyomatosis regressed during sirolimus therapy, but tended to increase in volume after therapy was stopped. Suppression of mTOR signaling might constitute an ameliorative treatment in patients with TSC or sporadic lymphangioleiomyomatosis.
The prognosis for patients with pulmonary TSC is generally one of progressive decline as a result of lymphatic obstruction, alveolar hyperplasia, pleural thickening, and cyst formation. In severe cases, progressive respiratory insufficiency and death may occur. These patients may be considered for lung transplantation, but they are not candidates, given the coexisting conditions from TSC.
Hereditary Hemorrhagic Telangiectasia
HHT, also known as Osler-Weber-Rendu syndrome, is an autosomal dominantly inherited condition characterized by telangiectasias of the skin and mucous membranes, and intermittent bleeding from vascular abnormalities. Angiomas of the skin and oral, nasal, and conjunctival mucosa become evident in the second or third decade of life. They appear bright red, punctate or linear, and blanch on pressure ( Fig. 12-7 ).

HHT occurs in 1 in 10,000 individuals and has wide ethnic and geographic distribution. Phenotypic heterogeneity exists with variable age of onset, organ involvement, and severity even within families. Two separate loci have been identified—endoglin ( HHT-1 ), on chromosome 9, and ALK-1 ( HHT-2 ), on chromosome 12—and this correlates with phenotypic variability. Both genes are members of the transforming growth factor-β receptor family.
HHT is the most common cause of pulmonary arteriovenous malformations (AVMs). Pulmonary AVMs occur in approximately 30% of affected individuals and may remain asymptomatic for many years. Fistulous vascular communications in the lungs may be large and localized, or smaller, multiple, and diffuse. They are frequently bilateral and have a predilection for the lower lobes. The usual communication is between the pulmonary artery and pulmonary vein; direct communication between pulmonary artery and left atrium is extremely rare. Desaturated blood in the pulmonary artery is shunted through the fistula into the pulmonary veins, bypassing the lungs, and entering the left side of the heart. This shunt may result in systemic arterial desaturation and cyanosis. The shunt across the fistula is of low pressure and resistance so that pulmonary arterial pressure is normal; cardiomegaly is not present. The electrocardiogram is normal.
The severity of pulmonary manifestations depends on the magnitude of the right-to-left shunting. Patients may present with dyspnea, exercise intolerance, and polycythemia. Hemoptysis is rare in children, but is the most common presenting symptom in adults. Physical findings include cyanosis, a systolic or continuous bruit over the site of the fistula, and digital clubbing. Features of HHT occur in about 50% of patients or other family members and include recurrent epistaxis and gastrointestinal tract bleeding. Recurrent epistaxis secondary to telangiectasia of the nasal septa and turbinates is the presenting sign in more than 50% of patients, and occurs in 95% at some point during the course of illness. Nosebleeds in patients with HHT, typically spontaneous and often nocturnal, have a mean age of onset of 12 years and a mean frequency of 18 episodes per month.
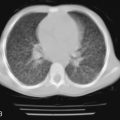
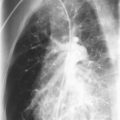
Chest radiographs may show oval or round, homogeneous, nodular opacities from a few millimeters to several centimeters in diameter owing to large fistulas. Pulmonary angiography with contrast enhancement usually reveals an artery entering the fistula and a vein leaving it ( Fig. 12-8 ). Multiple fistulas may be visualized on fluoroscopy as abnormal pulsations or on MRI. Selective pulmonary arteriography may be required to confirm site, extent, and distribution of fistulas and can confirm the diagnosis in virtually all cases.

In addition to pulmonary complications, patients with HHT may have AVMs of the brain. Brain and systemic abscesses are potentially serious complications in patients with HHT. Brain abscess can be the initial presentation in a patient with previously asymptomatic pulmonary AVM. Forty percent of patients with pulmonary AVMs may present with central nervous system manifestations, such as transient dizziness, diplopia, aphasia, motor weakness, or seizures. These findings may result from cerebral thrombosis, abscess, or paradoxical embolic events. Gastrointestinal bleeding secondary to telangiectasia occurs in 40% of patients, although rarely in children. AVMs also may manifest in the gastrointestinal tract, but are more typically seen in the liver. Hepatic AVMs, possibly more common in female patients, can lead to complications such as hemorrhage, portal hypertension, and cirrhosis ( Table 12-3 ).