Although hormones and growth factors have an influence on the proliferation of lung tissue, especially during early development, there are no significant alterations in lung function or respiratory morbidity associated with the most prevalent endocrine conditions in pediatric patients. Numerous hormones, including corticosteroids and the thyroid hormone, have been shown during the later stages of lung development to stimulate differentiation of type II alveolar epithelial cells and to produce architectural rearrangements of lung connective tissue elements that facilitate gas exchange. Glucocorticoids in the perinatal period delay Clara cell differentiation, elevate surfactant protein mRNA, and have a stimulatory effect on pulmonary cytochrome P-450. These effects translate into the known maturational changes induced by corticosteroids, and consequently they are used antenatally to prevent premature births.
This chapter reviews how endocrine disorders affect the respiratory system. Emphasis is placed on the pulmonary manifestations of diabetes, pseudohypoaldosteronism (PHA), hypothyroidism, hyperthyroidism, and obesity-hypoventilation syndrome. Clinicians should be aware of the potential relationships between endocrine disorders and pulmonary dysfunction.
Specific Disorders
Diabetes
Lung disease is not a major complication for children with diabetes. Barring an infection, it is not a likely threat even when the diabetes has reached a point where other complications are present. Nonetheless, several cross-sectional studies in patients with diabetes have found mild abnormalities in different lung function parameters. In one population cross-sectional study, Lange and colleagues found that forced vital capacity and forced expiratory volume in 1 second were significantly lower in individuals with diabetes than in nondiabetic controls. This finding was especially prominent among patients with insulin-dependent diabetes. Sandler and coworkers found mild but significant decreases in lung elastic recoil, diffusing lung capacity, and capillary blood volume in a group of patients with type 1 diabetes compared with healthy controls. The degree of pulmonary dysfunction correlated with the duration of the disease. Other investigators also have reported mild decreases in measures of lung function in patients with diabetes, but these results are hardly unanimous.
Experimental data from animal models of diabetes suggest that there are abnormalities in the lung connective tissue synthesis and turnover that are especially prominent in the absence of insulin replacement. Thickening of the alveolar wall from increased deposition of collagen fibers and a reduced degradation of connective tissue have been observed in rats made diabetic by treatment with streptozocin. This thickening also has often been noted in the alveolar and endothelial epithelial basement membranes of humans. Such abnormalities in the molecular composition of epithelial basement membranes are well-known complications of diabetes, and they have been extensively studied in the renal glomeruli, with excessive glycosylation of the collagen-like material that confirms the theory being proposed as the underlying pathophysiologic mechanism. As it pertains to the lung, it is possible that hyperglycemia leads to excessive nonenzymatic glycosylation of lung connective tissue, but the consequences of this remain unclear. Animal studies have reported discordant results. Some studies have described irreversible cross-linking of collagen (a form of the protein that is resistant to proteolysis), and a decreased activity of the enzyme calmodulin (which is involved in the regulation of connective tissue remodeling), whereas others have shown interference with the molecular cross-linking of elastin and collagen fibers. Regardless, this abnormal accumulation of collagen and elastin fibers alters the lungs’ mechanical properties, possibly explaining the decrease in lung tissue elasticity reported in clinical studies.
Notwithstanding the potential effects of diabetes on the lung, children with diabetic ketoacidosis who present with altered consciousness and vomiting are at an increased risk for aspiration. Because aspiration can lead to greater morbidity, the placement of a nasogastric tube and intubation in the presence of altered mental status should be approached with extra caution.
Diabetes is a common complication for patients with cystic fibrosis (CF), typically manifesting in the adolescent and adult years, although its occurrence has been reported in infancy. Because this particular diabetic condition has features of type 1 and type 2 diabetes, it has been named CF-related diabetes to distinguish it as a separate entity. Multiple studies have shown a clear correlation between the presence of CF-related diabetes and shortened survival and increased pulmonary morbidity. Finkelstein and associates reported a significantly shortened survival in CF patients with diabetes, with less than 25% surviving to age 30 years. In addition, clinical deterioration, as assessed by National Institutes of Health score, was apparent 2 years before the diagnosis of diabetes was made. Epidemiologic and registry studies concur in observing greater mortality and pulmonary morbidity among patients with CF-related diabetes. These patients are more likely to be malnourished and to have significant pulmonary dysfunction than CF patients without diabetes.
The underlying causes are still not well understood, but these studies raise the question whether it is more likely that a prediabetic state develops insidiously and then contributes to clinical decline, or, alternatively, that sicker patients are simply more susceptible to diabetes. Further longitudinal studies have shown a direct relationship between abnormal glucose tolerance and deteriorating pulmonary function. The annual rate of decline in pulmonary function over a 4-year observation period was found to have a direct correlation with the degree of glucose intolerance at baseline. Notably, the rate of pulmonary decline was inversely related to the magnitude of insulin secretion at baseline; this suggests a relationship between insulin deficiency and clinical deterioration. Because subjects at baseline were no different in terms of pulmonary function and nutritional status, these data strongly support the concept that the insulin-deficient state leads to progressively declining pulmonary function. In addition, female patients with CF-related diabetes have been reported to have worse pulmonary outcomes and an overall disadvantage in survival. The current recommendation is that CF patients older than age 13 should have annual screening for CF-related diabetes through oral glucose tolerance testing.
Infants born to mothers with diabetes are at increased risk for intrauterine or perinatal asphyxia. Maternal vascular disease, manifested primarily by nephropathy, may contribute to the development of fetal hypoxia and subsequent perinatal asphyxia. In addition, respiratory distress syndrome occurs more frequently in infants born to mothers with diabetes than in normal infants at each gestational stage, especially before 38.5 weeks. Hyperinsulinemia causes the delayed maturation of surfactant synthesis; a possible mechanism for this is by interfering with the induction of lung maturation by glucocorticoids. Infants born to mothers with diabetes also are at risk for respiratory morbidity from pneumonia, hypertrophic cardiomyopathy, and transient tachypnea of the newborn, which occurs two to three times more commonly in this group than in normal infants.
Growth Hormone Disorders
An increase in lung size has been reported in patients with acromegaly; however, this is not associated with any significant respiratory dysfunction. Obstructive sleep apnea (OSA) is most common in adult patients with either active or treated acromegaly. This condition is believed to be primarily a consequence of upper airway obstruction owing to hypertrophy of the tongue and pharyngeal tissues. In addition, growth hormone-induced changes in central respiratory control have been proposed as a possible mechanism for the disordered sleep pattern found in these patients.
Adrenal Disorders
Only one case has been reported of Cushing syndrome in an infant secondary to a pituitary adenoma who also presented with multicystic lesions in the lungs and kidneys. This infant had elevated serum cortisol levels and nonsuppressed adrenocorticotropic hormone levels and increased urinary steroids. The lung cysts were surgically removed and diagnosed as congenital benign lung cysts. This multicystic lung disease could simply be a coincidental finding because no other similar presentations have been described.
Pseudohypoaldosteronism
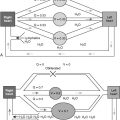
The content of body fluid Na + is tightly regulated by the epithelial Na + channel (ENaC), which is present in apical membranes of many Na + -absorptive epithelia, such as the renal tubules, distal colon, and lungs ( Fig. 8-1 ). ENaC is specifically inhibited by the diuretic amiloride, and it also is called the amiloride-sensitive Na + channel. The major physiologic role of ENaC is to maintain Na + homeostasis, blood volume, and blood pressure by providing an apical entry pathway for Na + ions to permit rapid transport of Na + from the luminal to the interstitial compartment. ENaC consists of three homologous subunits, α, β, and γ. The α subunit is essential for the formation of a functional ion channel, whereas the β and γ subunits can greatly potentiate the level of expressed Na + currents.

ENaCs belong to the Degenerin/ENaC superfamily of ion channels. The human ENaC genes have been cloned, and using genetic linkage analysis, it was found that the involvement of ENaC gene mutations results in two distinct human diseases. The autosomal dominant form affects the kidneys only (Liddle syndrome). It is characteristically free from any significant pulmonary involvement and is due to a defect in the function of the aldosterone receptor. The autosomal recessive form is a systemic syndrome that affects the kidneys, colon, sweat and salivary glands, and lungs. As mentioned before, this syndrome arises from abnormalities in the activity of ENaC. This channel is involved in the apical exchange of Na + and movement of water across mucosal surfaces, such as the airway surface. The state of hyperreninism and hyperaldosteronism in patients with systemic PHA type 1 is the result of sustained extracellular fluid volume depletion and is not due to peripheral resistance to mineralocorticoids.
Epithelial Na + Channel Mutations
Schaedel and associates looked for ENaC mutations in four patients with PHA type 1 and negative cystic fibrosis transmembrane regulator ( CFTR ) gene analyses: a 2-year-old girl with recurrent bronchopneumonia; an 8-year-old boy with frequent pneumonia colonized with Pseudomonas aeruginosa , normal pulmonary function tests, and a normal resting nasal potential difference; his deceased 7-week-old brother; and a 9-year-old boy with recurrent bronchopneumonia. All four patients had mutations of the ENaC α subunit (1449delC, 729delA, C1685→T). The authors suggested that an increase in the Na + concentration in the airway surface liquid probably promotes the growth of P. aeruginosa and reduces its killing, leading to P. aeruginosa lung disease. Lung involvement in patients with PHA type 1 seems to be related to mutations in the α subunit of ENaC, at least based on current evidence. Lung disease seems milder than in CF, however, with no reports of bronchiectasis in patients to date.
The clinical syndrome consists of urinary salt wasting, hyperkalemia, and metabolic acidosis associated with elevated plasma renin activity and aldosterone levels. These manifestations can be present in the neonatal period and lead to death from severe dehydration and electrolyte imbalance. Failure of lung fluid reabsorption at birth has been associated with neonatal respiratory distress syndrome in patients with PHA type 1 and related to ENaC, which is involved in this process. Additional clinical characteristics beyond the neonatal period include persistent clear nasal discharge, frequent lower respiratory infections associated with wheezing and crackles, and failure to thrive.
Hanukoglu and colleagues reported four patients with PHA type 1: an 8-year-old girl with frequent episodes of cough and lung crackles, who had a forced expiratory volume in 1 second of 91% predicted; an 8-year-old girl with recurrent lower respiratory tract infections and left lower lobe pneumonia, but normal pulmonary function; and twin 4-month-old boys with recurrent episodes of lower respiratory tract infection and wheezing. These patients had sweat chloride values ranging from 70 to 132. The authors noted that up to 4 years of age, respiratory infections usually occurred at times of dehydration. Later in life, the patients’ main symptoms were moderately severe cough, wheezing, and crackles.
Marthinsen and coworkers described a 6-year-old boy with PHA type 1. He had a sweat chloride value of 110, and a history of recurrent bronchopneumonia associated with dehydration. Since 18 months of age, sputum cultures were positive for P. aeruginosa . A computed tomography scan of his chest showed no evidence of bronchiectasis, and his pulmonary function tests were normal. He was treated with chest physiotherapy and inhaled antibiotics. MacLaughlin noted the similarities between CF and PHA type 1, in terms of pulmonary bacterial infection and sweat chloride concentration. Clinically, PHA type 1 can be indistinguishable from CF, unless serum aldosterone, plasma renin activity, and urinary electrolytes are measured, and genetic studies are performed to rule out the presence of CFTR mutations. These similarities are highlighted by the interaction of CFTR and ENaC. One of the physiologic functions of CFTR is to inhibit the ENaC. If CFTR is absent or expressed in reduced quantity, luminal Na + absorption through ENaC is enhanced ( Fig. 8-2 ). Na + absorption is osmotically drawing water from the airway surface liquid, contributing to depletion of airway surface liquid. Na + hyperabsorption could be an important factor in CF pathophysiology because mice overexpressing the β subunit of ENaC do exhibit signs of CF lung disease, such as mucous plugging and neutrophilic inflammation.

An alternative approach to CFTR pharmacotherapy would be to inhibit the enhanced Na + absorption through ENaC. In the nasal mucosa, the defect in Na + reabsorption can be shown by the absence of amiloride-sensitive Na + transport during nasal transepithelial potential difference measurements ( Fig. 8-3 ).

The pulmonary phenotype seems to be related to the absent or severely reduced Na + transport in airway epithelia, with failure to reabsorb fluid from the airway surface and presence of watery secretions. Despite frequent respiratory manifestations with respiratory exacerbations, patients with PHA type 1 do not experience the destructive process that patients with CF manifest as they age. In addition, airway colonization with pathogens is not as problematic as in patients with CF, which may explain the more benign course of their pulmonary disease.
At present, treatment of these patients is quite similar to the treatment of CF patients. Further research, looking particularly into the electrolyte composition of the airway surface liquid in CF patients, is needed to determine whether airway colonization with P. aeruginosa in these two conditions is related to common abnormalities in the electrolyte composition of the airway surface liquid or other host-related abnormalities in these two conditions. Whether patients with PHA type 1 and P. aeruginosa lung disease have mucoid Pseudomonas , and whether conversion to mucoid also is related to host and environmental factors is unknown.
Hypothyroidism
Many of the manifestations of hypothyroidism are a consequence of the hypometabolic state and accumulation of matrix glycosaminoglycans (myxedema) that are induced by the lack of thyroid hormone. The hypometabolic state is primarily manifested by weakness, fatigue, cold intolerance, constipation, weight gain, abnormalities in muscle tone, and bradycardia. Myxedema leads to coarseness and induration of the skin, puffy facies, enlargement of the tongue, and hoarseness. Specifically, from a respiratory perspective, respiratory muscle weakness manifests as hypoventilation with shortness of breath and decreased exercise capacity, although the presence of cardiovascular disease also could play a role. Abnormal ventilatory responses to hypoxia and hypercapnia can be shown in these patients as well, with the reduction in the ventilatory response to hypoxia being more pronounced. Thyroid hormone replacement therapy restores the normal response to hypoxia promptly, whereas the hypercapnic drive is slower to return to normal. The reason for the impairment of ventilatory responses in some hypothyroid patients is unknown, but may be related to the decrease in oxygen consumption associated with hypothyroidism.
Muscle weakness, impaired ventilatory responses, and potential upper airway obstruction determine the conditions for the development of significant sleep-disordered breathing. Multiple studies have shown the presence of sleep apnea with central or obstructive components. The obstructive form seems to be more common in these patients. OSA may be caused by an enlarged tongue or reduced oropharynx owing to mucopolysaccharide and protein deposition or by a myopathy involving the muscles of the upper airway. Overweight seems to compound this problem. Based on these findings, it has been proposed that screening for hypothyroidism should be considered during the evaluation of patients with sleep-disordered breathing. In the absence of clinical manifestations of hypothyroidism (i.e., subclinical hypothyroidism), the prevalence of sleep disorders is no different from euthyroid patients, and this needs to be taken into consideration to avoid unnecessary testing. The sleep-disordered breathing is reversible with the institution of appropriate therapy, although the obstructive component may take some time to revert completely.
Bilateral and unilateral pleural effusions have been reported in association with hypothyroidism. The nature of these effusions (transudate or exudate) and their causes is controversial. Changes in capillary permeability in the hypothyroid patient may be related to the development of pleural effusions, however.
The prevalence of hypothyroidism is high among patients with idiopathic pulmonary arterial hypertension, although hypothyroidism is not currently believed to be a risk factor for the condition. The basis of the observed association of the two disorders is unclear.
Hypothyroidism can cause abnormally high sweat electrolyte levels. The presence of elevated sweat electrolyte levels may lead to the misdiagnosis of CF during the evaluation of children with chronic illness. The elevated chloride concentrations return to normal with the start of replacement therapy. The abnormality in the ability of the sweat gland to secrete normal sweat is believed to be due to infiltration of the secretory coil with granular material.
Hyperthyroidism
The hyperthyroid state does not affect the lung directly, but the increase in metabolism and the development of a hyperthyroid myopathy can affect lung function and cardiopulmonary performance. The hypermetabolic state induced by hyperthyroidism increases the basal metabolic rate and has an associated increase in oxygen consumption and carbon dioxide production. Hyperthyroid patients are typically dyspneic, and show significantly lower resting arterial P co 2 and tidal volume with increased ventilatory responses to carbon dioxide and hypoxia. With exercise, even correcting for carbon dioxide consumption, further increases in ventilatory responses are noted, which are believed to be secondary to an increased central drive and can be blocked by β-blockers. A smaller increment in heart rate between rest and anaerobic threshold, a reduced oxygen consumption at anaerobic threshold, and a reduced oxygen pulse at anaerobic threshold also have been reported.
A small study in children with untreated hyperthyroidism found no significant differences at rest compared with a control group in oxygen uptake, minute ventilation, or respiratory rate. During exercise, there were significant differences, however, in oxygen uptake, heart rate, minute ventilation, and respiratory rate. Patients with thyrotoxicosis also have an increased resting reflex hypoxic drive to respiration. These findings suggest an inappropriate increase in respiratory drive, possibly secondary to increased adrenergic stimulation.
Abnormalities in lung function also have been reported in hyperthyroid patients, but a lack of correlation between the severity of dyspnea and abnormalities in lung function suggests that the dyspnea is probably due to extrapulmonary causes. The development of myopathy in the hyperthyroid state is known also to affect the respiratory muscles. This muscle weakness, in addition to the above-noted abnormalities, leads to an impaired exercise capacity and increases in breathing effort. In patients with thyrotoxicosis, a linear relationship has been shown between respiratory forces (maximum inspiratory pressure, maximum expiratory pressure) and levels of thyroid hormones (triiodothyronine, thyroxine). In thyrotoxicosis, respiratory muscle weakness affects inspiratory and expiratory muscles and contributes to the dyspnea and exercise abnormalities noted in these patients. Most of these abnormalities are reversible with therapy, although the pattern of breathing may not return to normal immediately.
Although animal studies suggested an effect of thyroid hormone on bronchial smooth muscle tone and contractility, hyperthyroidism might protect against carbachol-induced bronchospasm. In a study with normal volunteers with induced hyperthyroidism by administration of exogenous triiodothyronine for 3 weeks, no effect on airway reactivity or lung function was noted.
Neonatal Graves disease must be considered in any infant born to a woman with a history of Graves disease. Affected infants are often preterm or low birth weight, appear anxious, and are restless and irritable ( Fig. 8-4 ). They may be febrile, and often the skin is flushed and warm. Tachycardia is a consistent finding and may be accompanied by cardiomegaly and heart failure, with consequent respiratory distress. Thyromegaly is almost always present and sometimes may cause tracheal compression and added respiratory distress. Persistent pulmonary hypertension of the newborn with the characteristic severe respiratory failure also has been reported in these infants. It has been postulated that thyrotoxicosis has effects on pulmonary vascular maturation and the metabolism of endogenous pulmonary vasodilators, contributing to the development of persistent pulmonary hypertension of the newborn.