Infection in an immunocompromised child presents many diagnostic challenges. In patients with primary immunodeficiencies, the clinician is often faced with the difficulty of not only diagnosing the infectious agent in the lung but also determining whether this child with “too many” respiratory infections actually has an underlying immune problem predisposing to repeat or atypical infections. Many of the basic defects in patients with the primary immunodeficiencies come into play in those who are immunosuppressed by cancer chemotherapy or by specific antirejection therapies after organ transplantation: decreased number or function of B lymphocytes, T lymphocytes, and phagocytic cells as well. The conducting airways branch 20 to 25 times between the trachea and the alveoli. The large surface area of the conducting airways and alveolar surfaces (>70m in an adult) poses a great challenge for the lung defenses for even an immunocompetent host. The anatomy of the lung, lung products, cell receptors, and host cellular responses all contribute to the normal lung defense and disease prevention. A defect in any of these defenses can result in an increased susceptibility to infection.
This chapter first focuses on the primary immunodeficiencies and the associated cellular defects that predispose to pulmonary infections. In this section the discussion of roles of specific cell types in lung defenses provides background for the discussion of immune defects arising from cancer chemotherapy or use of immunosuppressive drugs after organ transplantation. Although this portion of the chapter cannot provide an exhaustive review of all pulmonary abnormalities in primary immunodeficiency states, we will also focus on the major immunodeficiencies as examples of alterations in the cellular immune system and their pulmonary manifestations. Readers are referred to several more recent reviews for more details on primary immunodeficiency states.
The focus of the chapter changes to the general principles underlying the immune and nonimmune pulmonary complications of cancer chemotherapy and immunosuppressive therapy after organ transplantation. Many of the cellular defects described in the section on primary immunodeficiencies come into play in those patients with secondary immunocompromise. The chapter concludes with a discussion of diagnostic tools available for defining the underlying causes of pulmonary abnormalities in patients with immunodeficiency or immunosuppression.
Primary Immunodeficiencies
This section focuses on the pulmonary manifestations of primary immunodeficiency diseases. More than 100 primary immunodeficiency diseases are well-characterized clinically or at the molecular level, or both. Antibody deficiencies are the most common primary immunodeficiency diseases, accounting for about 70% of cases. Patients with deficiencies in antibody production typically acquire infections from encapsulated ( Streptococcus pneumoniae, Haemophilus influenzae , and Staphylococcus species) and gram-negative ( Pseudomonas species) organisms. Chronic fungal and opportunistic infections are rare. Viral infections are handled normally, with the exception of enteroviruses, which can cause persistent meningoencephalitis.
In contrast, defects in T cell function lead to infections by viruses and opportunistic organisms. Affected infants can present early in life with chronic diarrhea and failure to thrive. Persistent infections with opportunistic organisms ( Candida albicans, Pneumocystis jiroveci [formerly P. carinii ]) and viral infections often can be fatal. Patients with T cell defects cannot reject allografts, placing them at risk for fatal graft-versus-host disease (GVHD) if they receive nonirradiated blood or blood product transfusions. Infants with severe combined immunodeficiency (SCID) have absent T cell and B cell function, placing them at risk for infections manifested by immunoglobulin and T cell defects. The knowledge of the genetic defects underlying these immunodeficiency states continues to grow; there are now more than 10 known gene alterations associated with the phenotype of SCID. As the molecular genetics of these diseases becomes better understood, the ability to treat the severe forms of primary immunodeficiency disease with stem cell transplant (SCT) or gene therapy has improved in recent years.
Deficiencies in Immunoglobulin Production
Overview
IgG and IgA are found in the epithelial airway–lining fluid and play important roles in lung defense against bacteria. Deficiencies in these antibodies occur in many primary immunodeficiencies and usually result in chronic sinopulmonary infections.
Secretory IgA is the predominant immunoglobulin isotype present in airway secretions. Secretory IgA serves several functions, including neutralization of viruses and exotoxin, enhancement of lactoferrin and lactoperoxidase activities, and inhibition of microbial growth. Because dimeric IgA is able to bind two antigens simultaneously, it is capable of forming large antigen-antibody complexes. In this manner, IgA neutralizes microbes, facilitates their removal by mucociliary clearance, inhibits microbial binding to epithelial cells, and inhibits uptake of potential allergens. Although concentrations of IgG in the upper airway are less than the concentrations of IgA, all IgG subclasses are detectable in respiratory secretions, and it is the primary antibody found in lower respiratory secretions. As opposed to IgA, which is actively transported into the airway, IgG reaches the airway largely by transudation through the mucosa. IgG functions by opsonizing microbes for phagocytosis and killing, activating the complement cascade, and neutralizing many bacterial endotoxins and viruses.
Deficiencies in IgA and IgG result in loss of mucosal protection against numerous pathogens. Selective IgA deficiency (defined by a serum IgA concentration <0.05mg/mL) may be asymptomatic; it is often detected in healthy individuals during routine blood donor screening. Symptomatic individuals present with various manifestations, including recurrent sinusitis, otitis media, pharyngitis, bronchitis, pneumonia, chronic diarrhea, and autoimmune syndromes. Individuals with associated IgE deficiency tend to have less serious pulmonary disease, in contrast to individuals with normal or high IgE, who in addition to the aforementioned disorders may have allergic respiratory problems and pulmonary hemosiderosis. IgA deficiency is associated with increased frequency of neoplastic and autoimmune disorders. IgG deficiency is associated with recurrent otitis media, sinusitis, bronchitis, and pneumonia. In addition, recurrence of airway infections may result in chronic airway injury with bronchiectasis more frequently in patients with IgG deficiency than in patients with isolated IgA deficiency. The combination of altered opsonic activity and bronchiectasis can result in chronic colonization with respiratory pathogens, such as Pseudomonas aeruginosa .
Compared with IgG and IgA, IgM seems to play little role in lung defense. Most IgM remains in the vascular space owing to its high molecular weight. IgM does gain access to the airway, however, by exudation or by active secretion via secretory components. IgM is capable of agglutinating bacteria and activating the complement cascade.
IgE seems to participate in immunity to parasites. It binds to the parasites, and eosinophils bind to the opsonized organisms via the IgE Fc receptors. Eosinophils are stimulated to release granular contents, resulting in lysis of the parasite. The major manifestation of the presence of IgE in the respiratory tract is related to its role in allergic disease.
Specific Diseases
Antibody production is altered in many primary immunodeficiency states ( Table 3-1 ). Severe recurrent infections associated with bronchiectasis occur with combined deficiencies in IgA and IgG production. X-linked agammaglobulinemia, the first primary immunodeficiency disease to be recognized, by Bruton in 1952, has been found to result from mutations in Bruton tyrosine kinase. Patients with X-linked agammaglobulinemia have a block in differentiation at all stages of B cell development, whereas T cell numbers and function are preserved. Initially, infants with X-linked agammaglobulinemia are protected by circulating maternal antibodies, but later develop recurrent sinopulmonary infections and may progress to bronchiectasis.
| DEFICIENCY | INHERITANCE | GENE LOCUS | GENETIC DEFECT | CELLULAR DEFECTS | CLINICAL FINDINGS |
|---|---|---|---|---|---|
| X-linked agammaglobulinemia (Bruton agammaglobulinemia) | X-linked | Xq21.3-Xq22 | Deficiency in Bruton tyrosine kinase | <1% circulating B cells | Recurrent sinopulmonary infections |
| Block in B cell differentiation | Most manifest in infancy, ~20% manifest in 3–5 yr old | ||||
| Deficiency in immunoglobulins of all types and isotypes | Bronchiectasis in right middle lobe and bases | ||||
| IgA deficiency | Variable (1/333-1/700 births) | 17p11 | TACI | Deficient isotype switch to IgA | Bacterial pneumonias, sinus disease |
| Possibly 6p21.3 | Others | Associated gastrointestinal infections | |||
| Increased atopy and neoplastic and autoimmune disorders | |||||
| Common variable immunodeficiency (likely several diseases) | AD with incomplete penetrance | 17p11.2 | TACI | Impaired production of all major antibody classes | Onset from early childhood to adulthood |
| 2q33 | ICOS | Absent immunoglobulins | Increased susceptibility to recurrent sinopulmonary infections | ||
| 16p11.2 | Others | Normal number of B cells | Leads to bronchiectasis | ||
| 22q13.1-22q12.21 | Failure to differentiate into antibody-secreting cells | Associated with autoimmunity in 20% | |||
| Abnormal T cells in 60% | |||||
| Autosomal recessive agammaglobulinemia | AR | Mutations in μ, α, λ5, BLNK , or LRRCE genes | All isotypes, decreased B cells | Severe bacterial infections | |
| Immunoglobulin heavy chain deletions | AR | Δ14q32 | Δ immunoglobulin heavy chains | Decreased IgG1, IgG2, IgG4, IgE, IgA1, IgA2 | Recurrent bacterial infections |
| ICOS deficiency | AR | 2q33 | Mutation in ICOS gene | Decreased all isotypes | Recurrent bacterial infections |
| Hyper-IgM syndrome | AR | 12p13 | Mutation in AID and UNG | Decreased IgG and IgA | Recurrent bacterial infections |
| 12q23-2q24.1 | Deficient B cell switching | ||||
| Normal T cells |
Similar to X-linked agammaglobulinemia, common variable immunodeficiency is characterized by impaired antibody production of all major classes. In contrast to X-linked agammaglobulinemia, patients with common variable immunodeficiency have normal numbers of circulating B cells; however, these B cells do not differentiate into antibody-secreting plasma cells. Common variable immunodeficiency affects males and females, and in some cases seems to be inherited in an autosomal dominant pattern with incomplete penetrance. Patients can present in infancy but may present in late childhood to adulthood. Common variable immunodeficiency likely represents several different genetic disorders.
Isolated IgA deficiency is the most common primary immunodeficiency disease, with an incidence of 1 in 333 to 1 in 700 in whites. The clinical manifestations of isolated IgA deficiency vary. Some affected patients are asymptomatic, whereas others can have recurrent respiratory and gastrointestinal infections, allergy, and increased incidence of autoimmune disease and malignancies. Because these patients can make IgG, however, bronchiectasis is uncommon compared with X-linked agammaglobulinemia or common variable immunodeficiency.
Isolated IgM deficiency is not associated with recurrent respiratory infections. Individuals with IgM deficiency seem to have a specific defect in B lymphocyte maturation, but the B lymphocytes are capable of secreting other antibody isotypes. The autosomal recessive form of hyper-IgM syndrome is caused by deficiency in nucleotide-modifying enzymes, UNG (uracil nucleoside glycosylase) and AID (activation-induced cytidine deaminase), expressed only in B cells that operate on sequential steps in antibody class switching. This form of hyper-IgM syndrome is accompanied by decreased levels of IgG and IgA, resulting in an increased propensity to bacterial infection (see Table 3-1 ).
Isolated IgE deficiency has not been reported. IgE deficiency in combination with IgG4 deficiency has been described in a patient who had recurrent otitis media and sinusitis. Hyper-IgE syndrome is discussed in the section focusing on phagocytic disorders.
Diagnosis
In patients with suspected immunoglobulin deficiency, quantitation of total serum IgA and IgG, and IgG subclasses should be performed along with measurement of the antibody response to protein (diphtheria and tetanus toxoids) and polysaccharide ( S. pneumoniae, H. influenzae, Neisseria meningitidis ) vaccines. In addition, flow cytometry to quantify B and T cell numbers and B and T cell stimulation studies should be done if immunoglobulin levels are low. Specific genetic studies can be done to identify patients with several of the immunoglobulin primary immunodeficiency diseases (see Table 3-1 ).
Treatment
IgG-deficient patients with recurrent respiratory tract infections often benefit from prophylactic antibiotics, intravenous gamma globulin therapy, and the use of airway clearance techniques. Patients with selective IgA deficiency are treated symptomatically for respiratory, gastrointestinal, and allergic problems. Because most preparations of gamma globulin contain IgA, the use of gamma globulin increases the risk of anaphylaxis if the recipient has anti-IgA antibodies. Transfusion of blood products presents a similar problem for these individuals. In patients with a combined IgA and IgG deficiency who need immunoglobulin therapy or the transfusion of blood products, it is crucial to ensure that the recipient does not have IgA antibodies or use a preparation that does not contain IgA.
Deficiencies in Cellular Immunity
Overview
Bronchial-associated lymphoid tissue functions in the development of adaptive lung defense. Bronchial-associated lymphoid tissue comprises intraepithelial lymphocytes, macrophages, dendritic cells, and natural killer (NK) and NK-T cells that recognize foreign substances, invading organisms or their exoproducts, and products of cell injury using innate receptor systems. The dendritic cells or macrophages interact with them and interact with lymphocytes to create the cellular immune responses, or to signal antibody production. These cells migrate to local lymph nodes, tonsils, or adenoids; process antigens; generate cytokines; and generate the adaptive immune responses. Extensive reviews of humoral (B cell mediated) and cellular (T cell mediated) immunity, antigen presentation, and cellular activation are well beyond the scope of this chapter; however, we briefly discuss the roles of T lymphocytes and cellular immunity with respect to lung defense.
Several types of T lymphocytes contribute to lung immunity and tissue pathology. Two major types of antigen receptors are used by T lymphocytes—the γδ T cell receptor (discussed later) and the αβ receptor (used by the CD4 + and CD8 + T cell populations). The αβ T cell mounts cytolytic responses to infected cells, makes cytokines, and stimulates B cell responses (see later). αβ T cells expressing the receptor protein, CD4, are termed T helper cells. CD4 + T cells recognize antigens presented via the MHC class II antigen and provide effector function primarily by the release of cytokines. T helper cells can be differentiated further phenotypically into Th1 and Th2 populations based on their profiles of cytokine production. Th1 cells differentiate in the presence of interleukin (IL)-12 and IL-18, and produce interferon-γ, IL-2, and tumor necrosis factor-α in response to antigen. These cells have been regarded as responsible for the delayed hypersensitivity response to viral or bacterial infection, stimulating local macrophage activation and neutrophil recruitment, and altering specific T cell responses. Th2 cells are driven to differentiate by the presence of IL-4, and in the presence of antigen they respond by making IL-4, IL-5, IL-10, and IL-13. These cytokines are responsible for driving B lymphocytes to make antibodies and lead to the recruitment and activation of basophils and eosinophils. The Th2 phenotype has been associated with an allergic/asthmatic phenotype in mouse models and human studies.
αβ T cells expressing CD8 are cytotoxic cells. In response to peptides presented by the MHC class I molecules, CD8 + T cells function in target cell toxicity. CD8 + T cell toxicity is mediated by the release of cellular granules containing perforin (perturbs the cell membrane) and granzymes (disrupt target cells by altering intracellular targets). In addition, CD8 + T cells can initiate apoptosis in target cells by Fas-FasL interactions. CD8 + cells reinforce viral defenses by rendering adjacent cells resistant to infection, presumably by release of interferons.
Three additional subsets of T cells contribute to innate lung responses, including recognition and elimination of tumor cells and certain pathogens using limited sets of conserved recognition receptors. NK cells are bone marrow–derived lymphocytes that are distinct from either B or T cells. NK-T cells are T cells that express the NK cell marker, NK1, a highly restricted/limited repertoire of the CD3/T cell receptor complex with specificity for antigens presented in association with CD1. These cells most closely resemble CD4 + T cells in terms of cytokine production. Finally, γδ T cells have a limited diversity of T cell receptors that recognize self and bacterial/protozoan antigens. These innate lymphocytes are considered to be a first line of defense against tumors and infection, and in modulating inflammation in the lung.
T cell responses are tightly regulated. T cell responses are necessary to eliminate pathogens and for immune memory; lack of T cell function can lead to serious life-threatening infections. Uncontrolled responses can cause autoimmune inflammatory diseases, however. In this section, we discuss primary immunodeficiency disease associated with T lymphocyte dysfunctions.
Specific Diseases
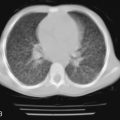
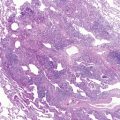
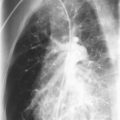
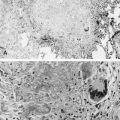
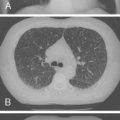
Mutations in the function of B cells or T cells result in immunodeficiencies of antibody production, cellular immunity, or both. The incidence of these deficiencies is unknown, but has been estimated to be 1 in 10,000 live births. Children with defects in T cell function can present with abnormal ability to limit “usual” childhood respiratory viral infections, which can be persistent or life-threatening (e.g., respiratory syncytial virus [RSV], parainfluenza virus, influenza virus, and adenovirus). Common childhood viral infections, such as varicella-zoster virus and measles, can cause serious lung infections in immunodeficient or immunosuppressed children. Children with T cell immunodeficiencies are more susceptible to opportunistic pathogens—agents that typically do not cause infections except in the context of immunodeficiency or immunosuppression, such as cytomegalovirus (CMV) and P. jiroveci ( Fig. 3-1 ). Children with T cell deficiencies can present in the first months of life with diarrhea and failure to thrive, or with persistent infections with Candida albicans, P. jiroveci, varicella-zoster virus, adenovirus, RSV, or CMV. Patients with T cell primary immunodeficiency disease may show abnormalities in antibody production because B cell function in antibody production is T cell–dependent. T cell primary immunodeficiency disease can manifest with bacterial infections as in patients with primary antibody deficiencies as described earlier.

Severe combined immunodeficiency (SCID) is an immunodeficiency characterized by a severe reduction in the number or function of T cells, which results in the absence of adaptive responses ( Table 3–2 ). This disease phenotype is the consequence of mutations in at least 10 different genes located on six different chromosomes (see Table 3-2 ). Four lymphocyte phenotypes have been identified associated with SCID, based on the effects of the mutation on T cell, B cell, and NK cell maturation (T − B + NK + , T − B + NK − , T − B − NK + , T − B − NK − ) (see Table 3-2 ). SCID causes severe infections with opportunistic infections in the neonatal period.
| DEFICIENCY | INHERITANCE | GENE LOCUS | GENETIC DEFECT | CELLULAR DEFECTS | CLINICAL FINDINGS |
|---|---|---|---|---|---|
| T − B + NK − SCID | |||||
| γc deficiency | XL | Xq13.1 | Mutations in common γ chain of IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 | Markedly decreased natural killer cells | Severe infections with opportunistic organisms soon after neonatal period |
| Markedly decreased T cells | Manifest with failure to thrive, chronic diarrhea, persistent thrush, pneumonia, sepsis | ||||
| Decreased serum immunoglobulin | |||||
| CD45 deficiency | AR | 1q31-1q32 | Mutation in CD45 gene | As per γc SCID | As above |
| Jak3 deficiency | AR | 19p13.1 | Janus kinase-3 deficiency ( Jak3 ) | As per γc SCID | As above |
| T − B + NK + SCID | |||||
| IL7Rα deficiency | AR | 5p13 | Mutation in IL7RA gene | Marked decrease in T cells | As above |
| Decreased serum immunoglobulin | |||||
| CD3δ deficiency | AR | 11q23 | Mutation in CD3D gene | As per IL7Rα | As above |
| CD3ϵ deficiency | AR | 11q23 | Mutation in CD3E gene | As per IL7Rα | As above |
| T − B − NK − SCID | |||||
| ADA deficiency | AR | 20q13.11 | Mutation in ADA gene | As per γc SCID | As above |
| Axial skeletal abnormalities | |||||
| T − B − NK + SCID | |||||
| RAG1/2 deficiency | AR | 11p13 | Mutation in RAG-1 or RAG-2 | Markedly decreased T and B cell numbers | As above |
| Decreased serum immunoglobulin | |||||
| Defective VDJ recombination | |||||
| Artemis deficiency | AR | 10p13 | Mutation in artemis gene | As above for RAG1/2 | As above |
| Other T Cell Defects | |||||
| X-linked hyper-IgM syndrome | XL | Xq26-Xq27 | Mutations in CD40 ligand (CD154) | Normal T cells | Neutropenia, thrombocytopenia, opportunistic infections |
| Only IgM and IgD bearing B cells | |||||
| Neutropenia | |||||
| CD8 deficiency | AR | 2q12 | Mutation in CD8A gene | Absent CD8, normal CD4 numbers | As for Artemis |
| TAP-1 and TAP-2 deficiency | AR | 6p21.3 | Mutation in TAP-1 or TAP-2 | As for CD8 deficiency | As for CD8, vasculitis occurs |
| DiGeorge syndrome | AD | 22q11.2 | TBX1 | Decreased or absent CD3 + cells | Cardiac and thymic defects, variable TCID |
Because of lack of graft rejection capability, these infants are at risk for GVHD if transfused with nonirradiated blood products. They are also at risk for severe systemic infection when immunized with live viruses (e.g., polio, measles, or varicella) or with bacille Calmette-Guérin. Diagnosis is possible at birth, with most affected infants having lymphopenia (<2000 lymphocytes/μL blood) and decreased in vitro proliferation studies. Even in the absence of a normal thymus, T cell development can be achieved after SCT, and this is the standard of care for these infants. Survival is improved if SCT occurs within the first 4 weeks of life. The improved survival of early transplants is attributed to the acquisition of opportunistic infections and their complications.
The X-linked form of hyper-IgM syndrome is caused by a defect in the CD40 ligand (CD40L) expressed by activated CD4 + T cells (see Table 3-2 ). The CD40-CD40L interaction is crucial in B cell–T cell signaling. A defect in this interaction results in normal to elevated IgM; reduced serum IgG, IgA, and IgE; and reduced memory B cells. In addition to the immunoglobulin defects, patients with X-linked hyper-IgM syndrome have opportunistic infections, neutropenia, thrombocytopenia, seronegative arthritis, inflammatory bowel disease, and greater likelihood of malignancies. The increased risk of opportunistic infections, autoimmune diseases, and malignancies is attributed to defective T cell–antigen-presenting cell interactions owing to the CD40L defect. P. jiroveci pneumonia has been reported in 40% of patients with mutations in CD40L.
The cellular immunodeficiency in DiGeorge syndrome results from primary T cell deficiency. DiGeorge syndrome is usually due to gene defects on chromosome 22, leading to abnormal development of the third and fourth pharyngeal pouches during embryogenesis. This leads to aberrant development of several organs, including the thymus, parathyroid glands, and heart. The degree of thymic hypoplasia varies, resulting in a variable severity in the T cell deficiency. In 80% of patients, the immunodeficiency is mild. Severe T cell deficiency and secondary deficient antibody responses can result from the thymic hypoplasia, however, leading to a severe disease phenotype (approaching SCID in severity). It is important to evaluate the presence of a thymus in patients with conotruncal cardiac defects and hypocalcemia because the T cell deficiency in DiGeorge syndrome places these patients at risk of GVHD after transfusion with nonirradiated blood products.
Not all T cell immunodeficiencies manifest with a severe propensity for infections. Isolated CD8 + T cell developmental and signaling defects can have a milder form of immunodeficiency. Mutations in the CD8α molecule prevent the final assembly of the CD8 + T cell receptor signaling complex. Mutations in the MHC-1 molecule (binds to the T cell receptor) caused by TAP1/TAP2 mutations or a mutation in the TAP-binding protein prevent normal antigen presentation to the T cell receptor. These mutations cause a deficiency in CD8 + T cell function but no deficiency in the CD4 + T cells, and lead to a milder disease phenotype (see Table 3-2 ). –
The dysregulation of cellular immunity can lead to immunodeficiency, autoimmunity, and malignancy, as described previously for X-linked hyper-IgM syndrome ( Table 3-3 ). Wiskott-Aldrich syndrome is an X-linked disorder characterized by eczema, thrombocytopenia with small defective platelets, and recurrent infections. Patients with Wiskott-Aldrich syndrome have impaired response to polysaccharides and protein antigens and aberrant T cell function. Systemic and chronic sinopulmonary infections develop in the first year of life. Pulmonary infections can be caused by encapsulated bacteria ( S. pneumoniae ), viruses (herpes simplex virus), or opportunistic organisms ( P. jiroveci ). Patients rarely survive beyond teenage years without SCT. Death usually results from infection, vasculitis, autoimmune cell cytopenias, or lymphoreticular malignancy.
| DEFICIENCY | INHERITANCE | GENE LOCUS | GENETIC DEFECT | CELLULAR DEFECTS | CLINICAL FINDINGS |
|---|---|---|---|---|---|
| Wiskott-Aldrich syndrome | XL | Xp11.22-11.23 | Mutation in WASP gene | Cytoskeletal defect affecting hematopoietic stem cell derivatives | Thrombocytopenia |
| Immunodeficiency | |||||
| Autoimmune disease | |||||
| Malignancy | |||||
| Progressive decrease in T cells | |||||
| Ataxia-telangiectasia | AR | 11q22-q23 | ATM | Disorder of cell cycle checkpoint leading to chromosomal instability | Thymic hypoplasia |
| Increased α-fetoprotein | |||||
| Telangiectasia | |||||
| Sensitivity to Ionizing Radiation | |||||
| Increased risk of malignancies, particularly lymphoid | |||||
| X-linked lymphoproliferative disease | XL | Xq25 | SH2D1A | Altered adapter protein regulating intracellular signaling | Uncontrolled T cell proliferation in Epstein-Barr virus infection, fatal mononucleosis, ineffective viral elimination, lymphoma, hypogammaglobulinemia |
| ALPS1a | AR | 10q24.1 | CD95 | Excessive CD4 − CD8 − αβ TCR + T cells | Autoimmunity |
| Defective lymphocyte apoptosis | Hypergammaglobulinemia | ||||
| Lymphoproliferation | |||||
| Increased risk of lymphoma | |||||
| ALPS1b | AR | 1q23 | CD95L | As above | As above, plus lupus syndrome |
| ALPS2b | AR | 2q33-2q34 | CASP8 | Defective lymphocyte apoptosis and activation | Recurrent bacterial and viral infections |
| Autoimmunity, hypergammaglobulinemia, lymphoproliferation |
Ataxia telangiectasia is another complex disease resulting from a disorder of cell cycle regulation that leads to chromosomal instability. Ataxia telangiectasia represents a combined immunodeficiency associated with neurologic, cutaneous, and immune abnormalities. These patients have recurrent sinopulmonary infection. Their cells have defective DNA repair and are sensitive to ionizing radiation. A concern is that repeated exposure to x-rays used in usual diagnostic studies places them at risk for lymphoreticular cancers and adenocarcinoma.
Several cellular defects can result in unchecked cell proliferation and susceptibility to infections and to tumors. X-linked lymphoproliferative disease is another example of immunodeficiency from aberrant cellular control (see Table 3-3 ). In X-linked lymphoproliferative disease, there is failure to control proliferation of cytotoxic T cells after infection with Epstein-Barr virus (EBV). The most common presentation is severe infectious mononucleosis, which is fatal in 80% of patients. The defect is in the gene encoding an adapter protein, SH2D1A, a protein that helps regulate the proliferation of T cells and NK cells. Autoimmune lymphoproliferative syndrome results from defects in cellular apoptosis (regulated cell death) mediated by CD95 (FAS). These patients have autoimmunity, hypergammaglobulinemia, lymphoproliferation, and excessive numbers of CD3 + , CD4 − , and CD8 − T lymphocytes (see Table 3-3 ).
Diagnosis
In an infant with possible T cell deficiency, total blood lymphocyte counts are a reasonable place to begin for diagnosis. Functional studies (immunoglobulin responses to protein and polysaccharide antigen immunizations), activation studies, and B, T, and NK cell markers can define the nature of the deficiency further. Finally, specific genetic studies can be conducted to identify several of the defined mutant genes.
Treatment
Treatment depends on the specific immunodeficiency. Prophylactic antibiotics, antiviral agents, or intravenous immunoglobulin can be used to prevent or ameliorate serious infections. For severe T cell deficiency, SCT early in life can be lifesaving. Thymic transplants have been performed to reconstitute the T cell defects in DiGeorge syndrome. Gene therapy has been investigated for specific primary immunodeficiency disease, but success has been tempered by the occurrence of leukemia in subjects resulting from the insertion of the retrovirus vectors near oncogenes. Newer vectors may improve the safety of this mode of therapy.
Deficiencies in Phagocyte Numbers, Function, and Opsonization
Overview
Neutrophils constitute about half the circulating white blood cell population, and their primary function is phagocytosis and killing of invading pathogens. For the neutrophil to accomplish this, it must respond to signals in the area of injury; adhere and transmigrate through the vascular endothelium; migrate to the area of infection; and recognize the pathogen, phagocytose, and kill it. Interruption of any of these steps would leave the host susceptible to infections. When the neutrophil has migrated into the tissue, its primary purpose is to recognize, ingest, and destroy pathogens. Phagocytosis comprises two steps: recognition and internalization of the foreign material into the phagosome. Killing or neutralization involves a secretory response. Materials may bind directly to the neutrophil, resulting in ingestion, or opsonization by serum proteins occurs. Neutrophils exhibit specific Fc-mediated binding and nonspecific binding using complement receptors CR1 and CR3. Intracellular killing generally is associated with the initiation of the respiratory burst. The importance of this process is shown by patients with chronic granulomatous disease (discussed later), whose neutrophils cannot undergo the oxidative burst. Deficiencies in neutrophil numbers, ability to migrate, opsonization, phagocytosis, or function can render the host susceptible to infection ( Table 3-4 ).
| DEFICIENCY | INHERITANCE | GENE LOCUS | GENETIC DEFECT | CELLULAR DEFECTS | CLINICAL FINDINGS |
|---|---|---|---|---|---|
| Neutrophil Numbers | |||||
| Kostmann syndrome | AD | 19p13.3 or 1p22 | Defects in ELA2 or Gfi1 | Mistrafficking or repression of neutrophil elastase | Neutropenia |
| Susceptibility to bacterial and fungal infections | |||||
| Congenital neutropenia | AD | 19p13.3 | ELA2 defect | As per Kostmann syndrome | As above |
| Cyclic neutropenia | AD | 19p13.3 | ELA2 defect | As per Kostmann syndrome | Susceptibility to infection at nadir of neutrophil counts |
| X-linked neutropenia | XL | Xp11.22-11.23 | WASP | Defective regulator of actin cytoskeleton | Neutropenia, infections as per Kostmann syndrome |
| Chemotaxis | |||||
| LAD1 | AR | 21q22.3 | INTG2 (CD18) | Defective neutrophil and lymphocyte migration from blood | Delayed umbilical stump separation, recurrent gingivitis |
| Lack CD18 | Absent tissue neutrophils, blood neutrophilia | ||||
| Function | |||||
| Hyper-IgE syndrome | ADv | 4q21 | Unknown | Unknown | High IgE and eosinophilia |
| Skin abscesses, pneumonia with pneumatocele formation | |||||
| CGD | AD | Xp23 | CYBB;gp91 phox | Absent cytochrome b ~70% of cases | Granulomatous lesions of lung, skin, lymph nodes, and liver: Staphylococcus aureus, Burkholderia cepacia, Serratia marcescens, Nocardia and Aspergillus species |
| XL | 16q24 | CYBA;p22 phox | Absent cytochrome b <5% of cases | ||
| AR | 7q11.23 | NCF1;p67 phox | Cytochrome positive | ||
| 1q225 | NCF2;p67 phox | ||||
| IFNγR1 deficiency | AR, AD | 6q23 | IfnγR1 | Loss of IFN-γ binding | Severe infections: Salmonella , HSV, CMV, parainfluenza, RSV, mycobacteria |
| IFNγR2 deficiency | AR, AD | 21q22 | IfnγR2 | Loss of IFN-γ binding | As above |
| Opsonization | |||||
| Complement C2 | AR | 6p21.3 | C2 defect | Absent complement activity | Pyogenic infection, vasculitis, SLE-like |
| Complement C3 | AR | 19p13.3-p13.2 | C3 defect | As above | Recurrent pyogenic infections |
| MBL deficiency | AR | 10q11.2-q21 | MBL2 | Deficiency in MBL2 | Increased pyogenic infections and sepsis |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree