LEARNING OBJECTIVES
1. Recognize central, segmental, and subsegmental pulmonary emboli on chest-computed tomography (CT).
2. Define the role of ventilation–perfusion scintigraphy, CT pulmonary angiography, chest magnetic resonance imaging/magnetic resonance angiography, CT venography, and lower extremity venous ultrasound studies in the evaluation of a patient with suspected venous thromboembolic disease, including the advantages and limitations of each modality depending on patient presentation.
3. Recognize enlarged pulmonary arteries on a chest radiograph and distinguish them from enlarged hilar lymph nodes.
4. Recognize enlargement of the central pulmonary arteries with diminution of the peripheral pulmonary arteries on a chest radiograph and suggest the diagnosis of pulmonary arterial hypertension.
5. Name several causes of precapillary and postcapillary pulmonary arterial hypertension.
Pulmonary vascular disease is a relatively common cause of chest pain and dyspnea. It can be acute, as in pulmonary embolism (PE), or chronic, as in most cases of pulmonary arterial hypertension (PAH). This chapter will review these two conditions and pulmonary artery tumors. Pulmonary vasculitides are discussed in Chapter 4. Pulmonary arteriovenous malformations are discussed in Chapter 16.
PULMONARY THROMBOEMBOLIC DISEASE
PE is the third most common acute cardiovascular disease, after myocardial infarction and stroke (1). However, there is considerable uncertainty and confusion with regard to accurate diagnosis of this condition. The clinical signs and symptoms associated with PE are nonspecific, as are laboratory investigations, electrocardiograms, and chest radiographs. When PE occurs without infarction, the chest radiograph may be normal, or it may show any or all of the following: oligemia of the affected lung (the Westermark sign; see Fig. 2.27), increase in the size of the main pulmonary artery, elevation of the diaphragm, pleural effusion (usually small and unilateral), focal consolidation (from hemorrhage), or discoid atelectasis. The chest radiograph is usually abnormal in patients with PE; however, with nonspecific subsegmental atelectasis being the most common abnormal finding (2). No chest radiographic sign is specific for PE or infarction, and the sensitivity of chest radiography for these conditions is poor. Even with a large pulmonary artery clot burden, the chest radiograph can be normal (3). The main role of the chest radiograph, therefore, is to exclude other diagnoses that might mimic PE clinically, such as pneumonia or pneumothorax. Because PE often goes undetected, the diagnosis of PE should be considered in any patient who presents with acute shortness of breath, tachycardia, and pleuritic chest pain.
Deep venous thrombosis (DVT) originates most commonly in lower extremity or pelvic veins, where they dislodge and propagate cranially into the pulmonary arterial tree. Radiologic studies used to diagnose thromboembolic disease include chest radiography, ventilation–perfusion (V/Q) scans, computed tomographic pulmonary angiography (CTPA), magnetic resonance imaging/magnetic resonance angiography (MRI/MRA), CT venography (CTV), MR venography, and lower extremity ultrasound. Once the gold standard for diagnosing PE, catheter-based pulmonary angiography has been replaced by CTPA. The ideal test to diagnose PE should be accurate, direct (objective), rapid, safe, readily available, and of reasonable cost. Because only approximately one-third of patients with symptomatic venous thromboembolism manifest PE (4), a diagnostic test that is able to provide information regarding the presence and significance of other chest disease would also be desirable. None of the common tests in use (other than CTPA) meet all or even most of these criteria. V/Q scintigraphy was the main imaging modality used in the evaluation of patients with suspected PE until the advent of multidetector CT scanning. A high-probability V/Q scan provides sufficient certainty to confirm the diagnosis of PE, while a normal or near normal scan reliably excludes the diagnosis. However, in the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED) study (4), indeterminate scans, which were present in 364 (39%) of 931 patients, showed a 30% incidence of PE, and low-probability scans (i.e., two thirds of V/Q scans in the PIOPED study) were not useful in establishing or excluding PE. Since the introduction of multidetector CT with high-spatial and temporal resolution, CTPA has become the method of choice for imaging the pulmonary vasculature when PE is suspected in routine clinical practice (5).
The sensitivity and specificity of multidetector CTPA to diagnose PE vary between 83% and 100% and 89% and 97%, respectively (5). The advent of multidetector CT scanners has improved the visualization of the segmental and subsegmental arteries, with subsequent improvement in the depiction of peripheral PE. The reported frequencies of isolated subsegmental PE range from 1.0% to 5.4% (5). The clinical relevance of small peripheral pulmonary emboli and the need to administer anticoagulants in such cases remain a subject of debate (Fig. 17.1). In certain circumstances, patients with isolated subsegmental PE (i.e., good cardiopulmonary reserve, absence of coexisting DVT or thrombophilia) may not need anticoagulation. The risk of anticoagulation may exceed the risk of morbidity and mortality from the suspected clot in this setting (6). In such cases, negative findings on a lower extremity study are mandatory before deciding to withhold anticoagulation. Several investigations have found the negative predictive value of CTPA to be ≥97% (7), suggesting that anticoagulants can be safely withheld when CTPA is normal and of good diagnostic quality. The possibility of using ECG-gated CT angiography for simultaneous assessment of coronary artery disease, aortic disease, and PE as a potential cause for chest pain or dyspnea could improve patient evaluation and triage, especially in the emergency department.
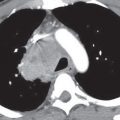
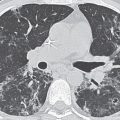
Characteristic CTPA findings of acute PE are: (a) partial central filling defect surrounded by a thin rim of contrast material, or (b) complete filling defect with obstruction of an entire vessel section (“vessel cutoff sign”) (Figs. 17.2 to 17.6). Pulmonary arteries that are completely obstructed by an acute embolus usually have an increased diameter (Figs. 17.7 and 17.8). Arteries peripheral to a central thrombus may or may not opacify. Central clot does not necessarily completely obstruct the distal flow of contrast. Although nonocclusive clot is depicted by CTPA, false-negative scintigraphy in this setting is well known. Acute embolic obstruction of a large degree of the pulmonary circulation increases pulmonary vascular resistance, leading to acute PAH. CTPA findings suggesting this complication include right ventricular enlargement (right ventricle/left ventricle ratio >1) and straightening or leftward bowing of the interventricular septum (Figs. 17.9 and 17.10) and reflux of iodinated contrast into the inferior vena cava. Pitfalls to be aware of in diagnosing PE include lymph nodes, impacted bronchi (Figs. 17.11 and 17.12); respiratory motion, vessel bifurcation, unopacified pulmonary veins (Fig. 17.13); periarterial abnormalities (lymph node enlargement or infiltration of the axial interstitium by edema fluid, inflammation, or neoplasm), pulmonary artery catheters, and pulmonary artery sarcoma (8).
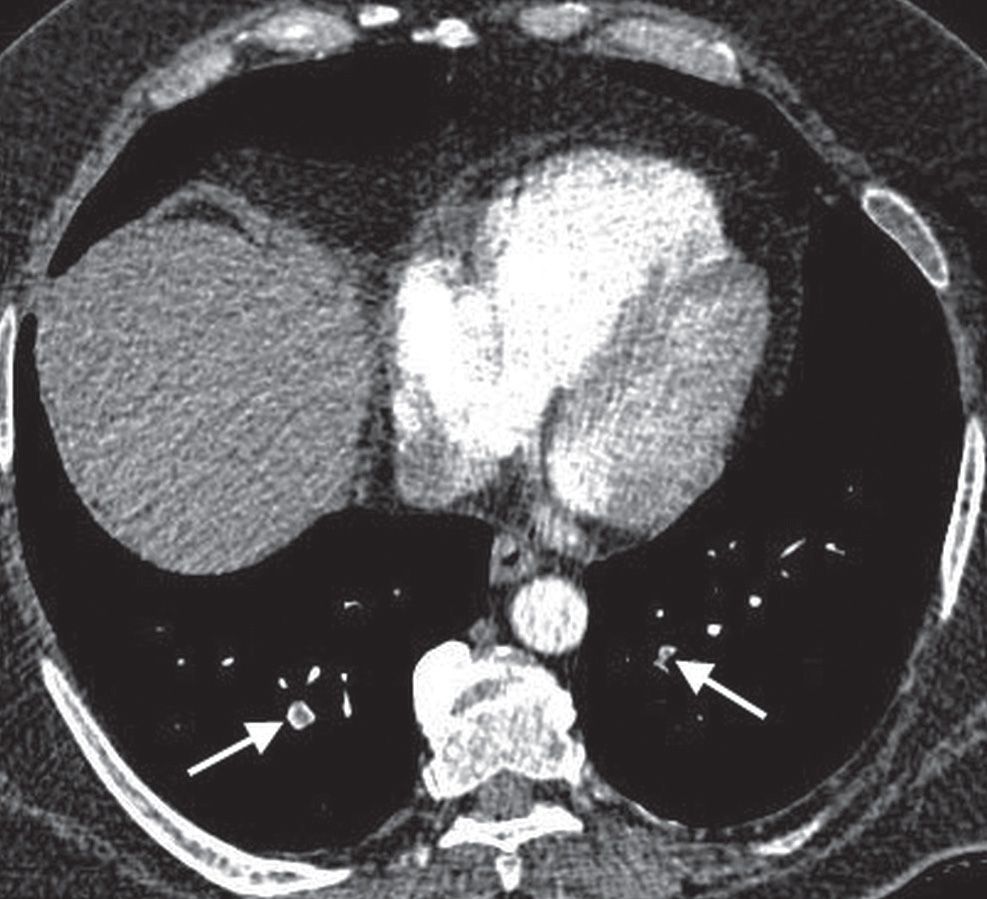
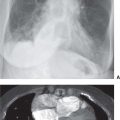
FIG. 17.1 • Subsegmental PE. CT of a 55-year-old man with acute shortness of breath shows subsegmental PE in both lower lobes (arrows).
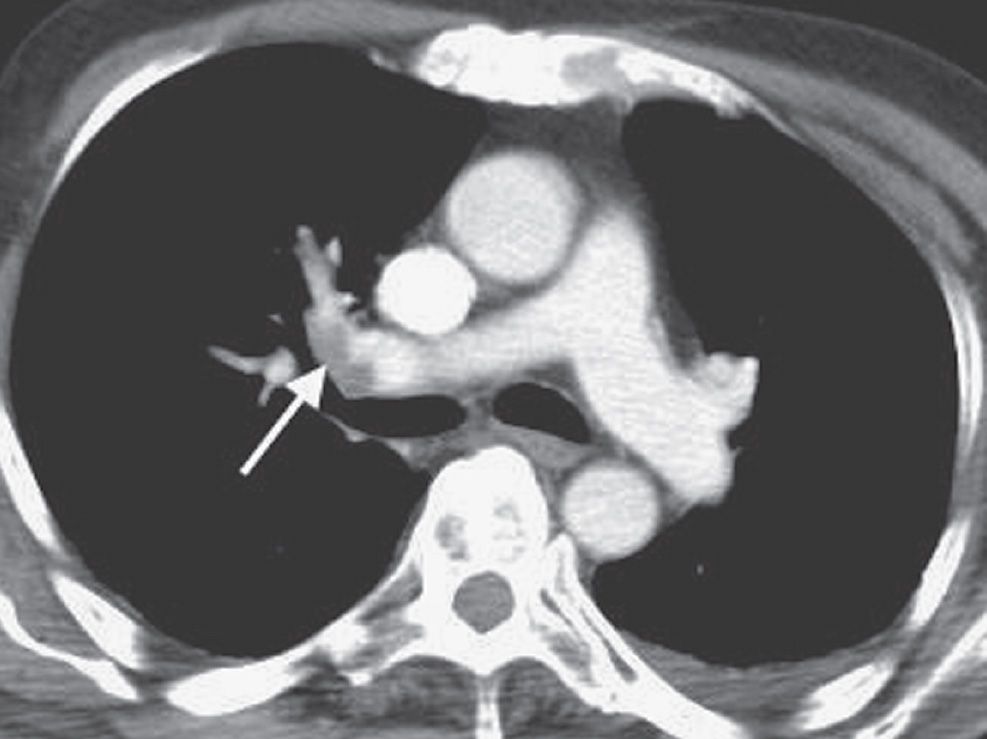
FIG. 17.2 • Incidental PE on CT. CT of a 70-year-old man with colon cancer shows intraluminal filling defect (arrow) in the right upper lobe pulmonary artery. The study was performed to assess for metastatic disease. Acute emboli are occasionally detected incidentally on routine CT; such findings illustrate the importance of evaluating the pulmonary arteries on all CT studies.
CTPA findings diagnostic of chronic PE include mural thrombus (adherent to the arterial wall), which may or may not be calcified (Figs. 17.14 and 17.15); webs, stenosis, or strictures of the arteries (Fig. 17.16); and a central “dot” of contrast surrounded by circumferential thrombus, which is indicative of recanalization. Ancillary findings include mosaic perfusion with decreased caliber of vessels in the hypoattenuated areas of lung (Fig. 17.17; see also Fig. 2.48), enlarged pulmonary arteries and right ventricle (Figs. 17.18 and 17.19), and enlarged bronchial arteries (Fig. 17.20). CTPA, like conventional angiography, usually enables distinction between acute and chronic PE; this is not possible with scintigraphy.
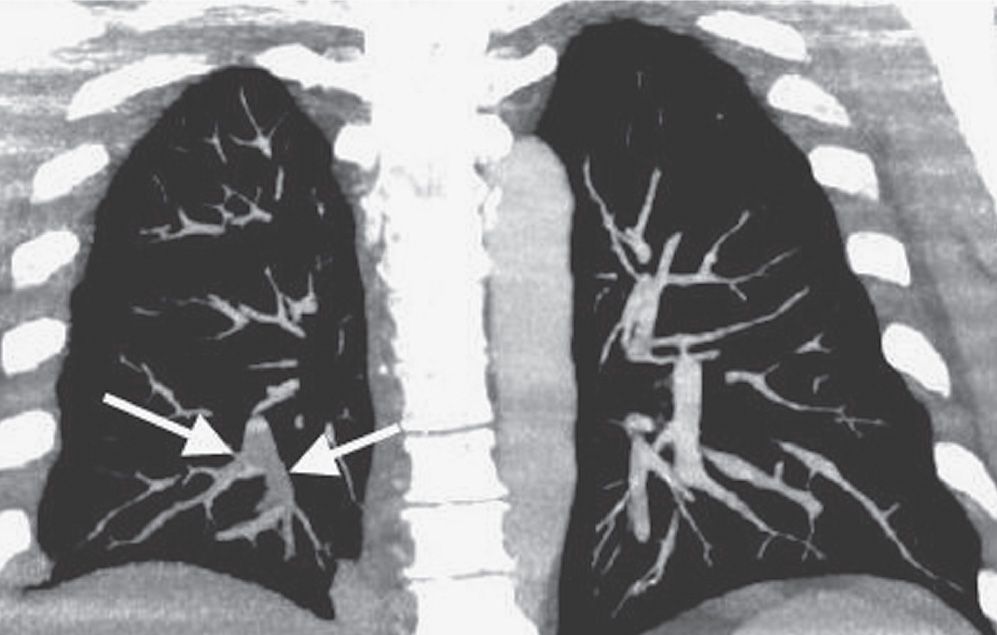
FIG. 17.3 • Acute PE. Coronal CTPA of a 43-year-old man with acute shortness of breath shows extensive intraluminal filling defect within the right lower lobe pulmonary arteries (arrows).
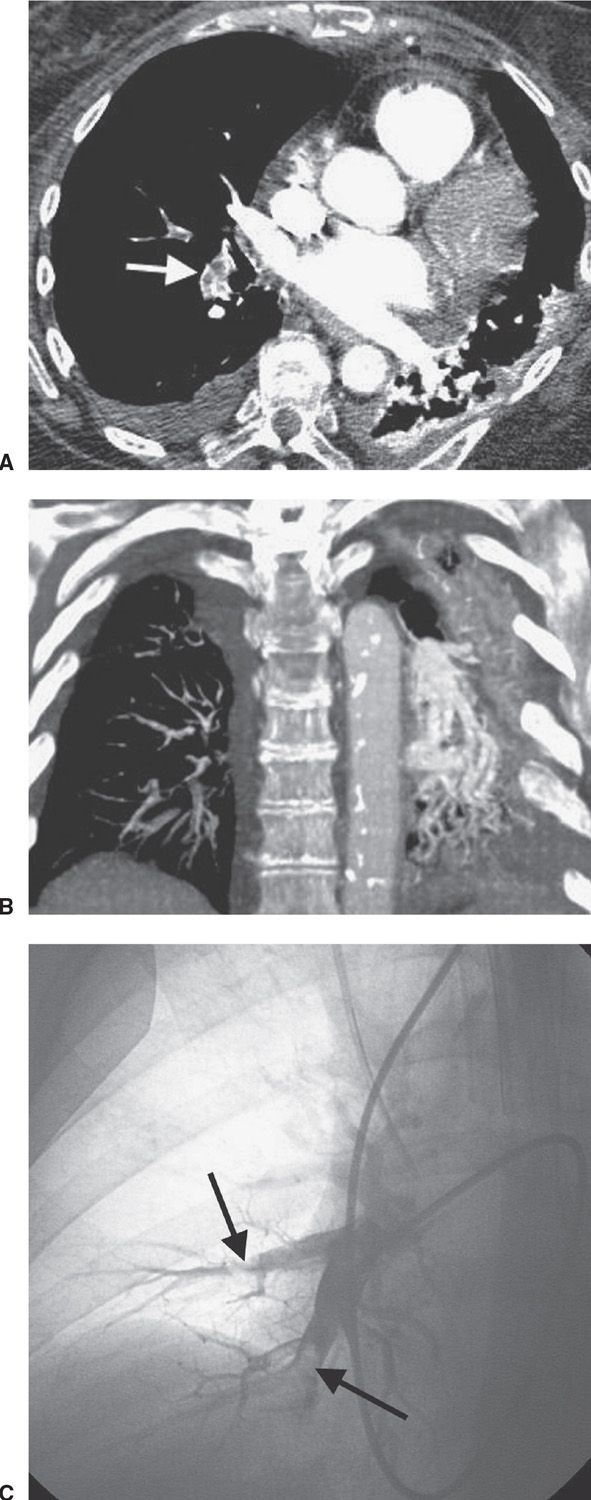
FIG. 17.4 • Acute PE. A: CTPA of a 77-year-old man with shortness of breath shows intraluminal filling defects, surrounded by a rim of contrast, within the right lower lobe segmental pulmonary arteries (arrow). B: Coronal CTPA shows decreased caliber of arteries in the right lung compared with the left and filling defect within right lower lobe vessels. C: Catheter-based pulmonary angiogram confirms clot within right lower lobe vessels (arrows).
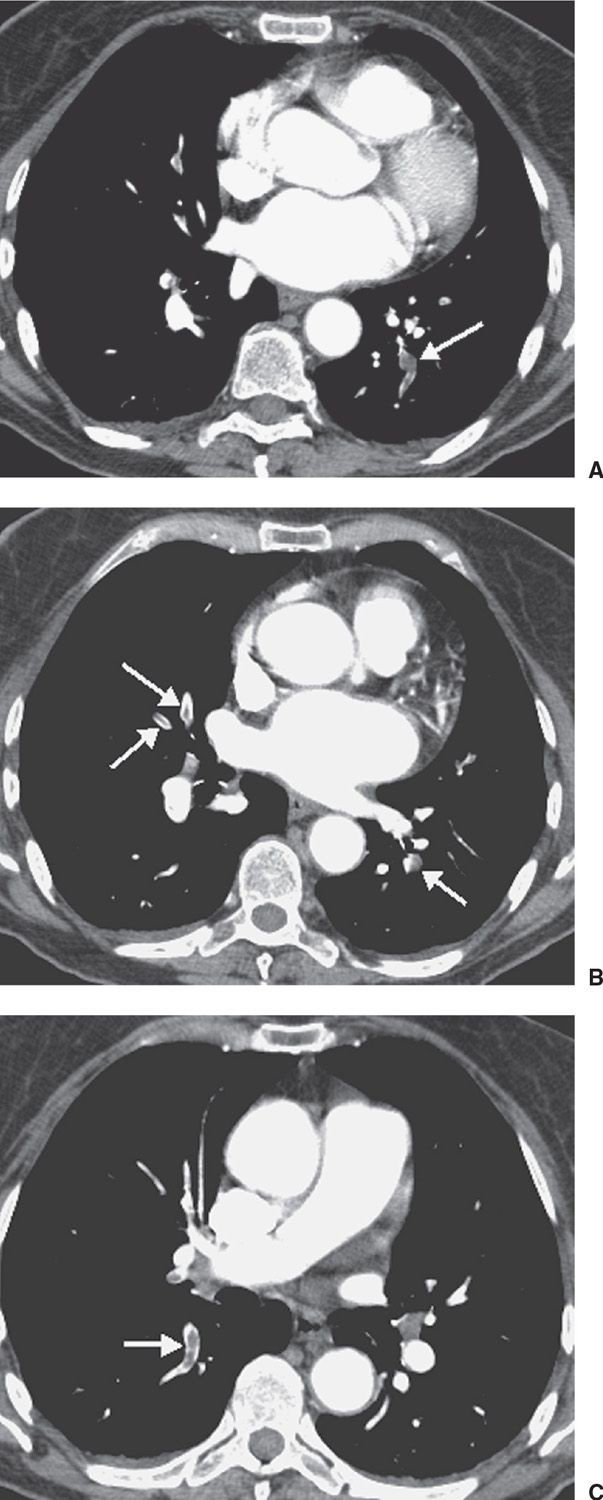
FIG. 17.5 • Acute PE. A: CTPA of a 77-year-old woman with a gastrointestinal bleed and DVT shows an intraluminal filling defect in a left lower lobe segmental pulmonary artery (arrow). B: CTPA at level superior to (A) shows intraluminal filling defects, surrounded by contrast material, in the right middle lobe and left lower lobe pulmonary arteries (arrows). C: CTPA at a level superior to (B) shows an intraluminal filling defect, surrounded by a thin rim of contrast material, in a right lower lobe segmental pulmonary artery (arrow).
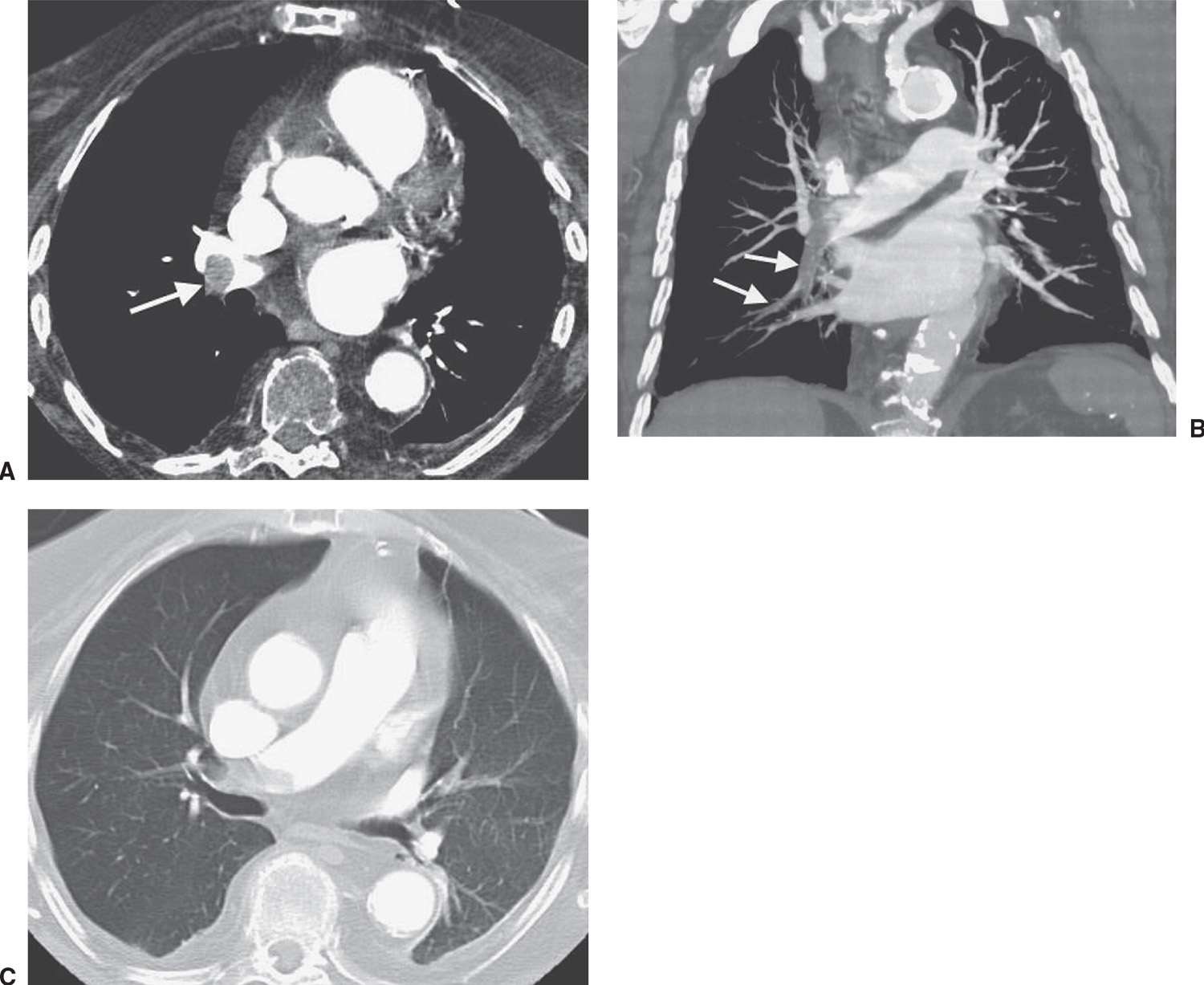
FIG. 17.6 • ACUTE PE. A: CTPA of a 78-year-old woman shows an intraluminal filling defect surrounded by contrast material in the proximal right lower lobe pulmonary artery (arrow). B: Coronal CTPA shows that the intraluminal filling defect extends from the proximal right lower lobe pulmonary artery inferiorly to distal branches (arrows). C: CTPA with lung windowing shows oligemia and diminution of vessels on the right (Westermark sign).

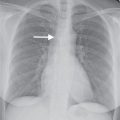
FIG. 17.7 • Acute PE. A: Posteroanterior (PA) chest radiograph of a 52-year-old woman with cholangiocarcinoma shows a rounded opacity at the left costophrenic angle, representing a Hampton hump of pulmonary infarction. B: CTPA shows a saddle embolus bridging the lingular and left lower lobe pulmonary arteries (arrow). C: CTPA at a level inferior to (B) shows intraluminal filling defects expanding the proximal lower lobe pulmonary arteries (arrows).
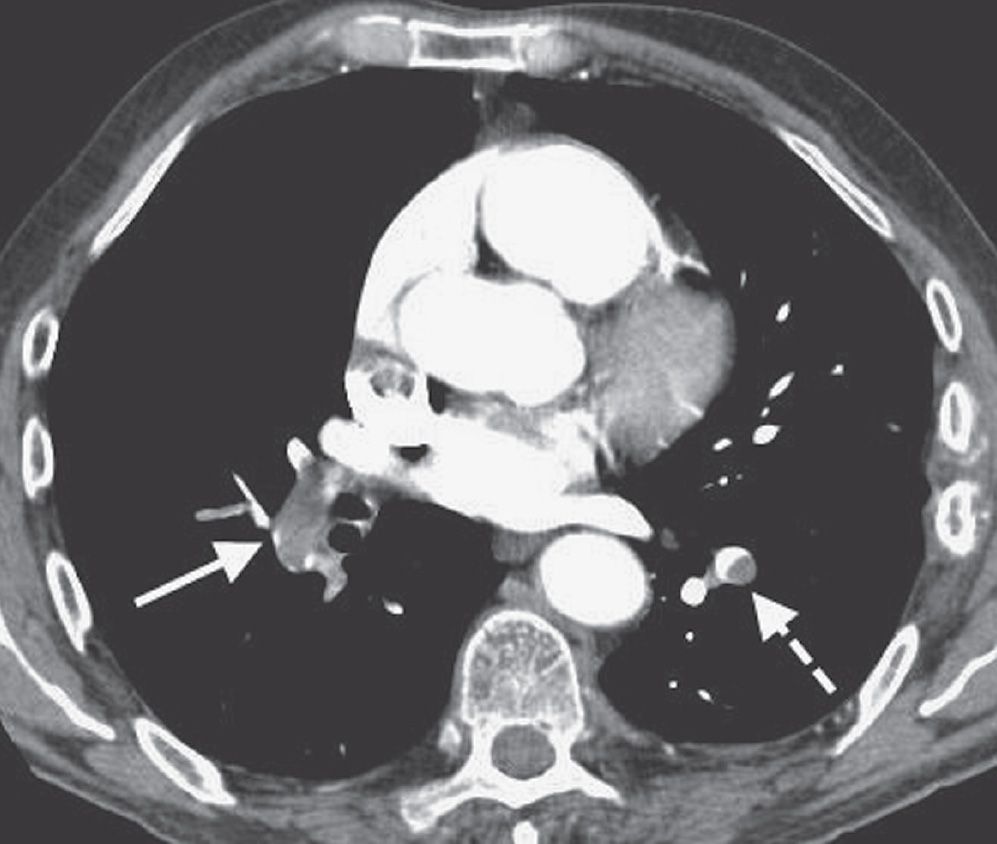
FIG. 17.8 • Acute PE. CTPA of a 76-year-old man with acute shortness of breath shows a large intraluminal filling defect within the proximal right lower lobe pulmonary artery (solid arrow) and a smaller intraluminal filling defect within a segmental pulmonary artery to the left lower lobe (dashed arrow).
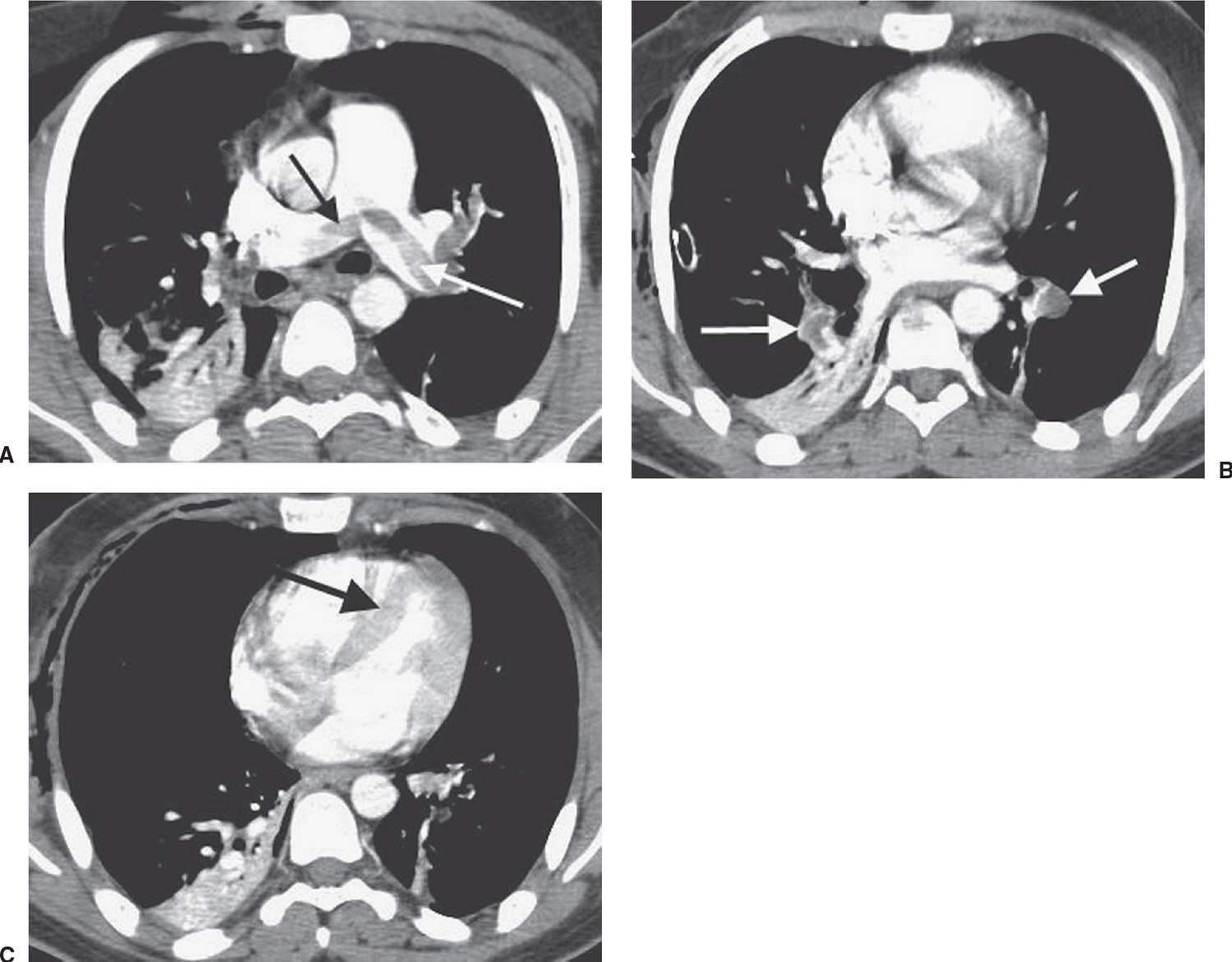
FIG. 17.9 • Acute PE associated with pulmonary arterial hypertension. A: CTPA of a 23-year-old man involved in a motor vehicle crash shows a saddle embolus straddling the right and left main pulmonary arteries (arrows). The central pulmonary arteries are enlarged. B: CTPA at a level inferior to (A) shows thrombus within segmental branches of the lower lobe pulmonary arteries (arrows). C: CTPA at a level inferior to (B) shows leftward bowing of the interventricular septum (arrow).

FIG. 17.10 • Acute PE associated with pulmonary arterial hypertension. A: CTPA of a 23-year-old woman with acute shortness of breath shows filling defects within the right and left pulmonary arteries (arrows). B: CTPA at a level inferior to (A) shows bilateral intraluminal filling defects and associated expansion of the involved arteries (arrows). C: CTPA at a level inferior to (B) shows enlargement of the right ventricle and leftward bowing of the interventricular septum (arrow).
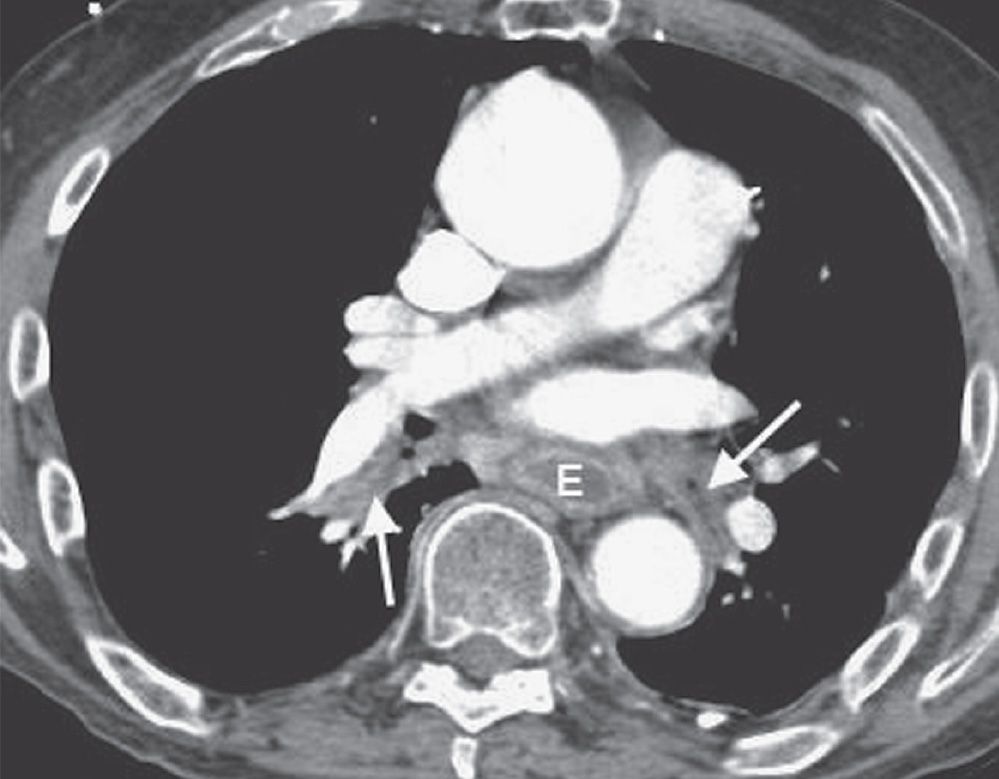
FIG. 17.11 • Mucous plugging. CTPA of a 75-year-old man with an esophageal stricture and gastroesophageal reflux shows a dilated esophagus (E) and low-attenuation material within the lower lobe segmental bronchi (arrows). The adjacent pulmonary vessels enhance normally.
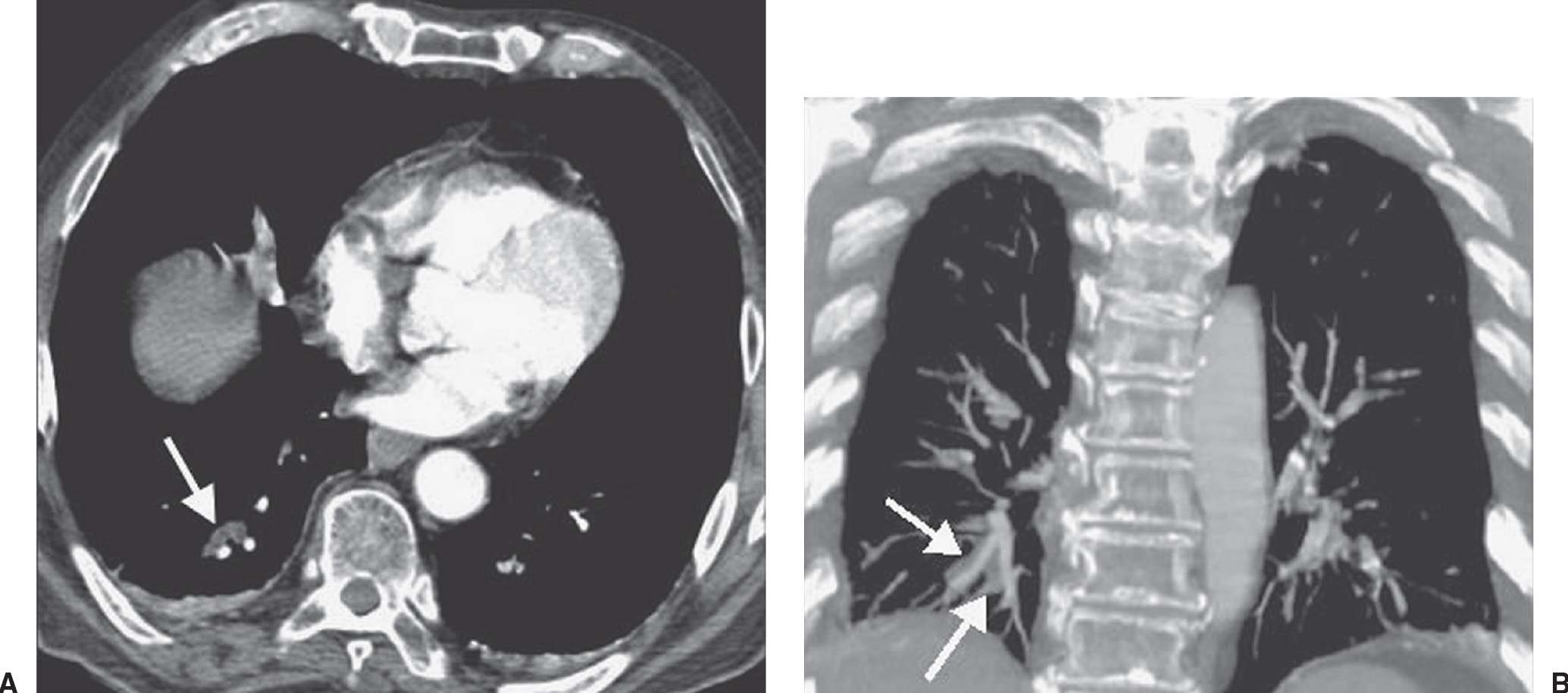
FIG. 17.12 • Mucous plugging. A: CTPA shows low-attenuation material occluding the right lower lobe subsegmental bronchi (arrow). The adjacent pulmonary vessels enhance normally. B: Coronal CT shows the impacted right lower lobe bronchi (arrows) adjacent to normally enhancing pulmonary vessels.
Major advantages of CTPA over V/Q scintigraphy to investigate patients suspected of acute PE include (a) direct visualization of emboli on CTPA; (b) evaluation of the lung parenchyma and mediastinum, which may provide an alternate diagnosis; and (c) capability of acquiring a CTV study without additional contrast (Fig. 17.21). Investigators have shown that, in addition to incidental findings, CTPA provides an alternative diagnosis (e.g., pneumonia, pneumothorax, pleural effusion, pericarditis, aortic dissection, aortic aneurysm, congestive heart failure, rib fracture, lung nodules or mass, mediastinal mass or air, gallstones, and chronic obstructive pulmonary disease) in up to two-thirds of patients with an initial suspicion of PE (9). Limitations of CTPA include patients with an allergy to contrast material, impaired renal function, the inability to lie supine, the inability to be transported to the CT scanner, or inadequate intravenous access. Other limitations of CTPA include motion artifact caused by inability of the patient to hold his or her breath or by adjacent cardiac motion, poor contrast bolus enhancement, image noise in large patients, partial volume averaging (Fig. 17.22), parenchymal disease, and streak artifact from lines and tubes or dense contrast material.
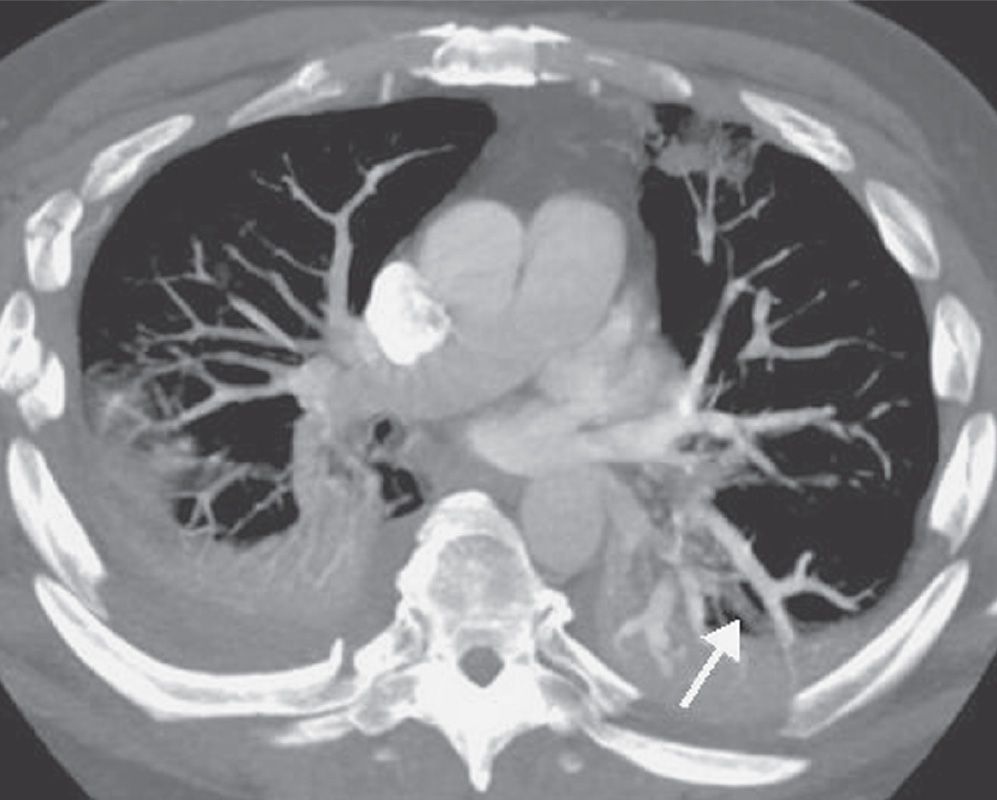
FIG. 17.13 • Pulmonary vein. CTPA shows a nonenhancing pulmonary vein in the left lower lobe (arrow). This should not be confused with a pulmonary artery. Pulmonary veins can be traced back to the left atrium on serial images.
PE and DVT are different manifestations of the same clinical disease. One advantage of CTPA is the ability to add CTV, to detect DVT in the legs and pelvis (Figs. 17.23 and 17.24). Both CTPA and CTV can be accomplished with the same bolus of contrast agent. Unlike lower extremity ultrasound, CTV can image the external and internal iliac veins. There is continued debate regarding the use of CTV. Several studies in which single-detector and multidetector CT angiography were used have shown that the addition of CTV to the CTPA study increases the percentage of patients requiring anticoagulation by 5% to 27% (5). However, less than 1.5% of patients with a negative CTPA or V/Q scan who have not received anticoagulants develop clinical evidence of PE within the next 3 months (5). In general, the use of CTV is recommended when emphasis must be placed on a complete vascular examination that can be accomplished expeditiously (5). When there are concerns about radiation exposure, CTV can be substituted with lower extremity US. CTV of only the femoral and popliteal veins, excluding the pelvic veins, can reduce radiation exposure. When evaluation of the lower extremity veins is not important clinically, CTV can be omitted. Venous thrombosis can also occur in the upper extremities and in the thorax and can be detected on CTPA (Fig. 17.25).
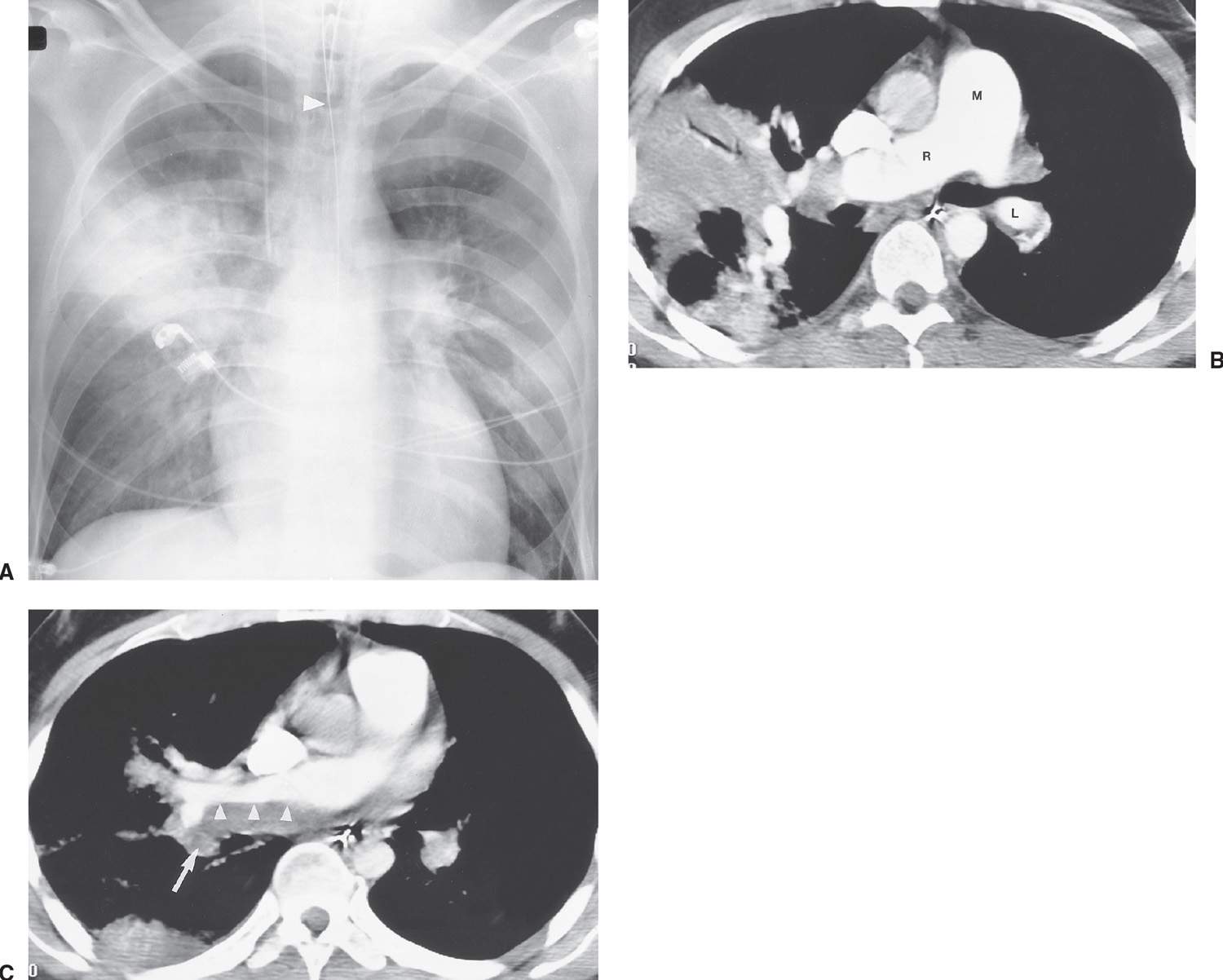
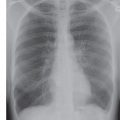
FIG. 17.14 • Acute and chronic PE. A: Anteroposterior recumbent chest radiograph of a 27-year-old man with a history of DVT and acute shortness of breath shows right upper lobe airspace disease, mimicking pneumonia, and fullness of the left hilum, mimicking adenopathy. Endotracheal tube is positioned slightly high (arrowhead). B: CTPA shows wedge-shaped, pleural-based airspace disease in the right upper and lower lobes. The main (M), right (R), and left lower lobe (L) pulmonary arteries are enlarged, correlating with the measured systolic pulmonary artery pressure of 90 mm Hg. C: CTPA at a level inferior to (B) shows old low-attenuation clot, eccentrically distributed along the posterior wall of the right pulmonary artery (arrowheads), and acute clot filling a right lower lobe basilar segmental pulmonary artery branch (arrow).

FIG. 17.15 • Chronic PE. A: CTPA of a 62-year-old woman with metastatic pancreatic cancer shows eccentric filling defect within the right lower lobe segmental pulmonary arteries (arrow). B: CTPA at a level inferior to (A) shows eccentric filling defect within a subsegmental right lower lobe branch (arrow).
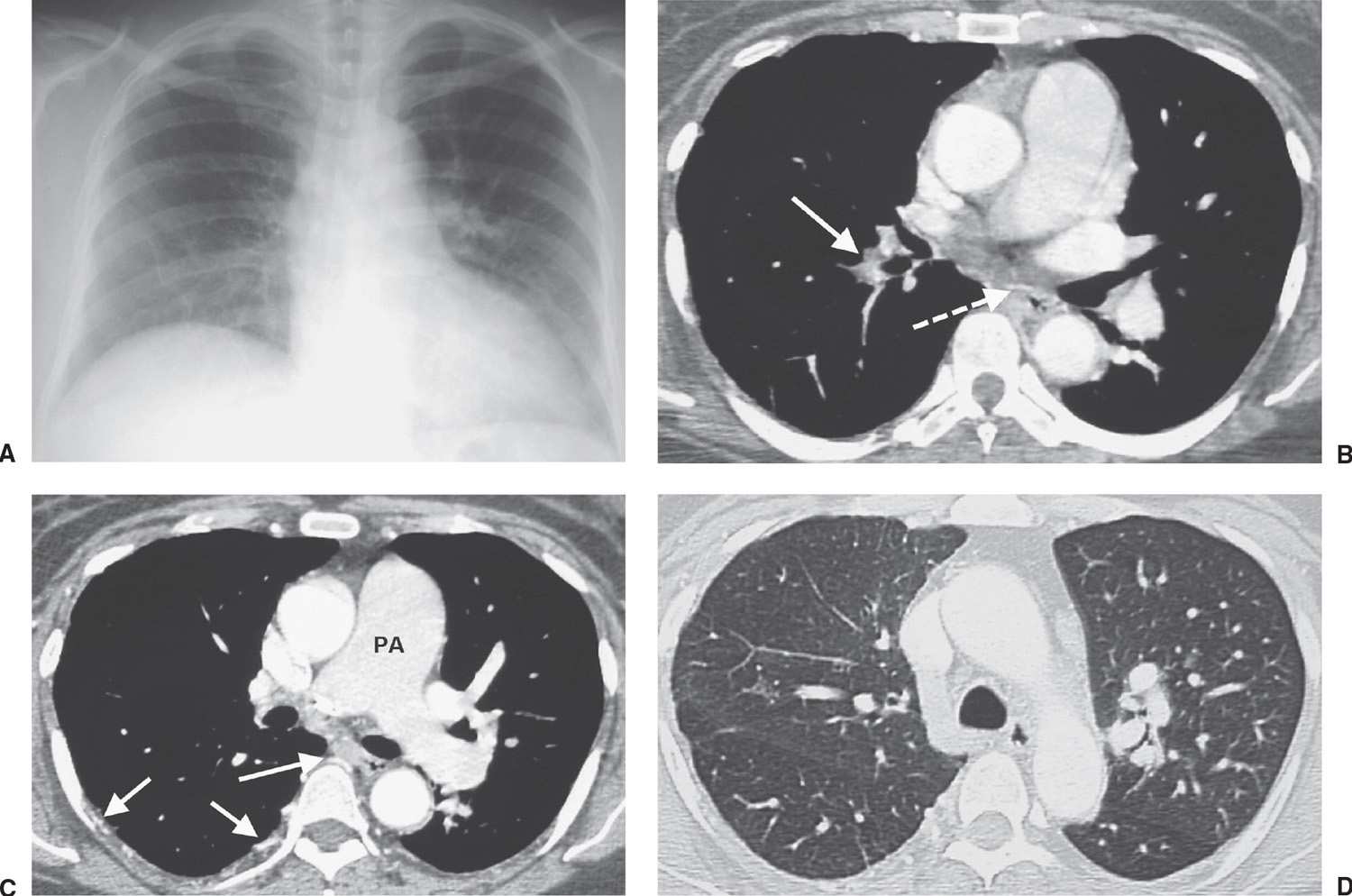
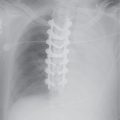
FIG. 17.16 • Chronic PE. A: PA chest radiograph of a 43-year-old woman with recurrent DVT and PE for 20 years shows a small right pulmonary artery and diminutive vessels in the right upper lobe. B: CTPA shows a small irregular right pulmonary artery with residual clot and areas of recanalization (solid arrow) and bronchial artery collaterals (dashed arrow). C: CTPA at a level inferior to (B) shows additional bronchial artery collaterals in a paraspinal and subpleural location (arrows). The main pulmonary artery (PA) is markedly enlarged. D: CTPA with lung windowing shows small right pulmonary arteries and a mosaic pattern of lung attenuation.
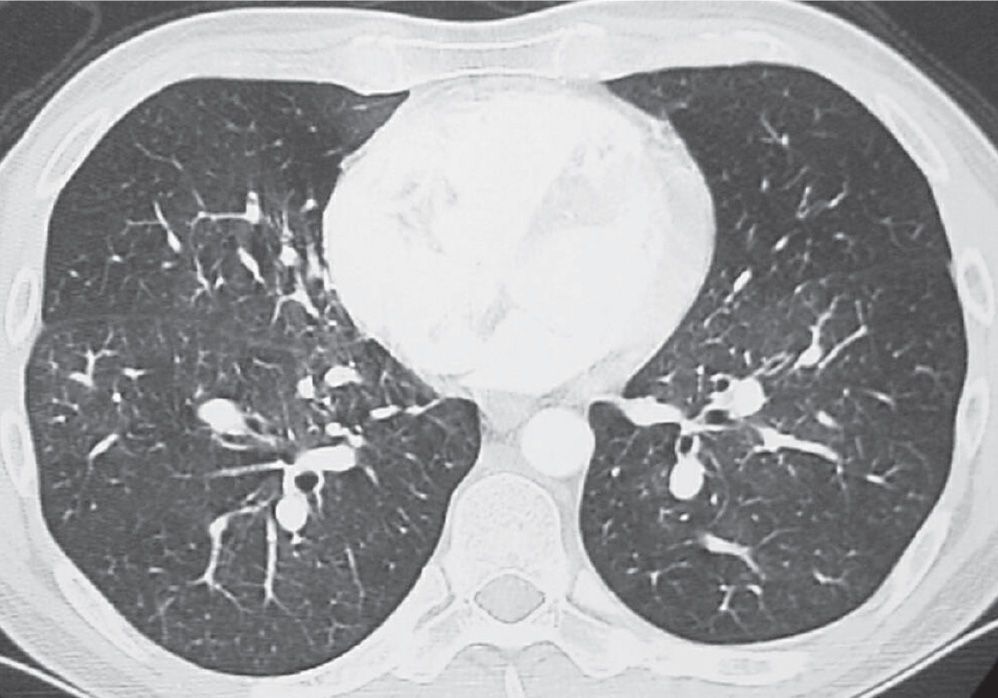
FIG. 17.17 • Chronic PE. CTPA shows a mosaic pattern of lung attenuation. Note diminutive vessels in the areas of hypoattenuated lung.
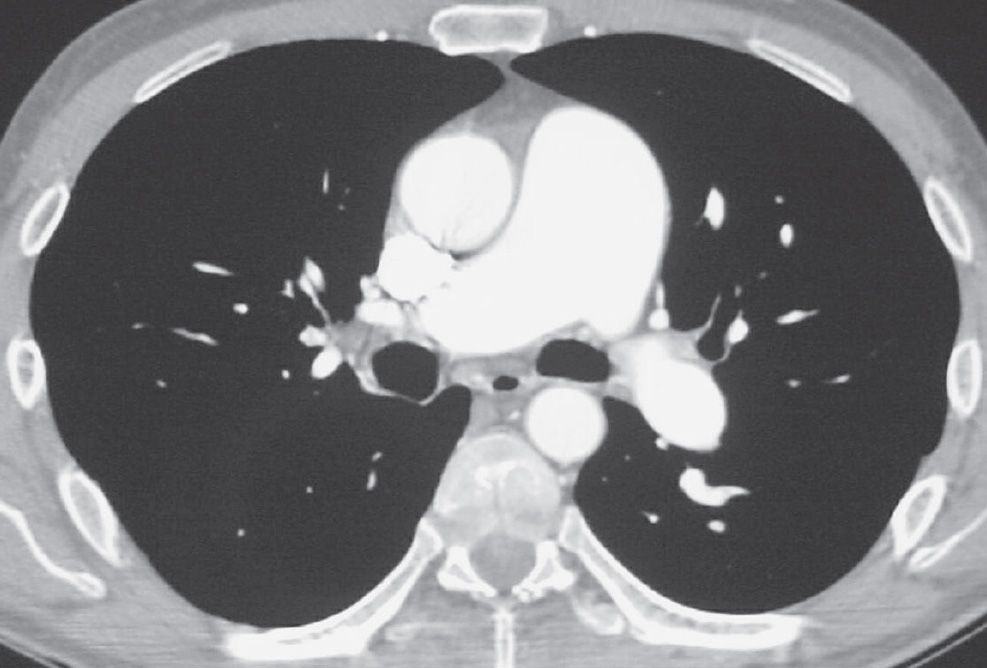
FIG. 17.18 • Chronic PE. CTPA shows marked enlargement of the main pulmonary artery, which is larger in diameter than the adjacent ascending aorta.
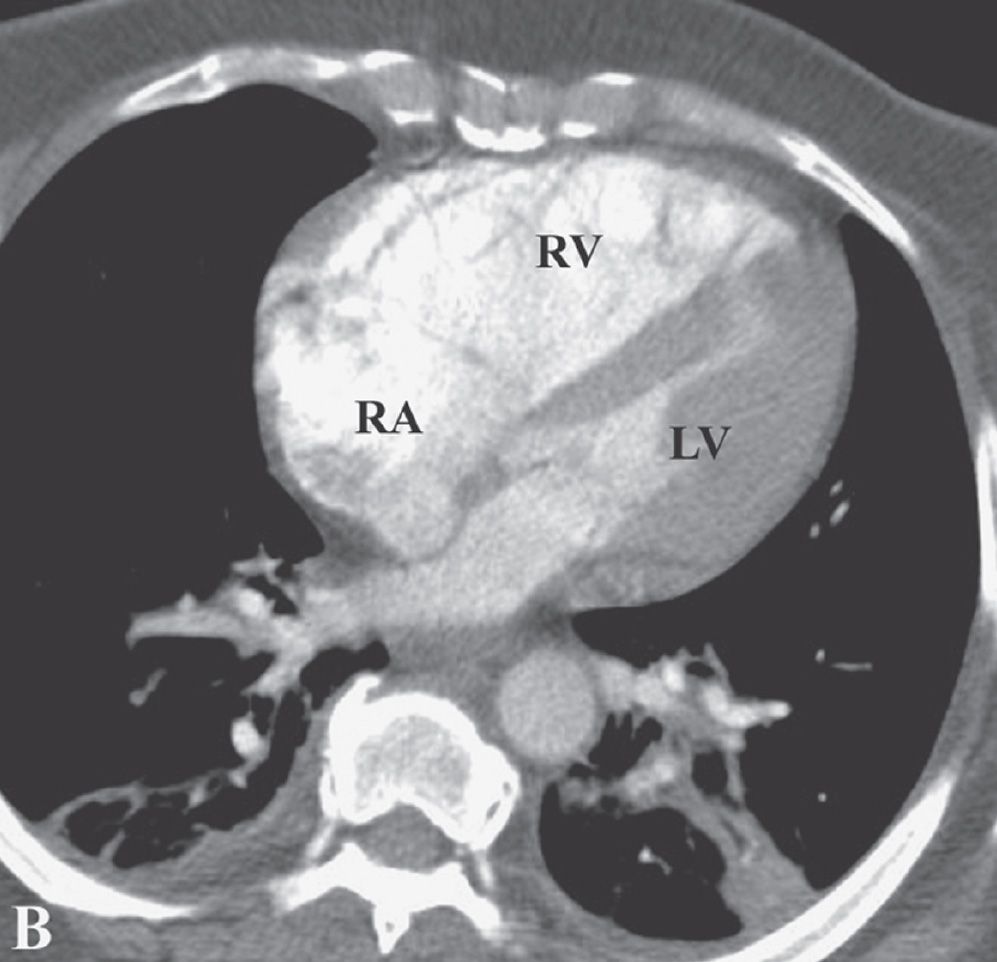
FIG. 17.19 • Chronic PE. CTPA shows enlargement of the right ventricle (RV) and right atrium (RA). The right ventricle/left ventricle (LV) ratio is greater than 1.
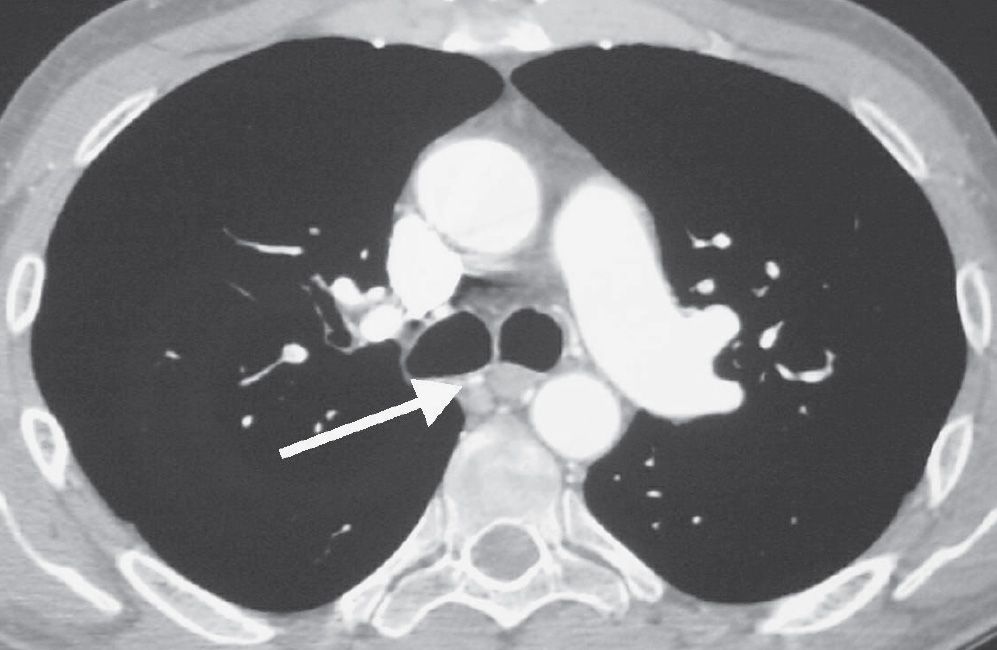
FIG. 17.20 • Chronic PE. CTPA shows enlarged bronchial arteries (arrow) adjacent to the esophagus.
The D-dimer assay, a test that detects one of the products of fibrin breakdown in the blood, is an important rapid initial test for DVT and PE. Recent studies show that the enzyme-linked immunosorbent assay (ELISA) D-dimer test can accurately rule out DVT and PE in the vast majority of cases (6). However, this test can be falsely positive in postoperative patients, patients on anticoagulation, and patients with recent trauma.
MRI is useful in the evaluation of suspected PE when patients are allergic to iodinated contrast medium. Because it does not involve ionizing radiation, it is also advantageous in children and pregnant women. The sensitivity of MR is 100% for PE in the central and lobar arteries, 84% in the segmental arteries, but only 40% in the subsegmental branches (5). MR angiography of the pulmonary arteries and MR venography for DVT performed with state-of-the-art techniques can potentially serve as a second-line examination in the evaluation of suspected acute PE in patients who are unable to receive iodinated contrast material for CT or for whom ionizing radiation is of concern. However, MR examinations are more complex and less robust, examination times are longer, and patient access to MR is more limited. Furthermore, the limited ability to detect pulmonary disorders other than PE, and alternate diagnoses, is a serious disadvantage compared with CT. Patients with a pacemaker or various implanted devices are largely excluded from MR.
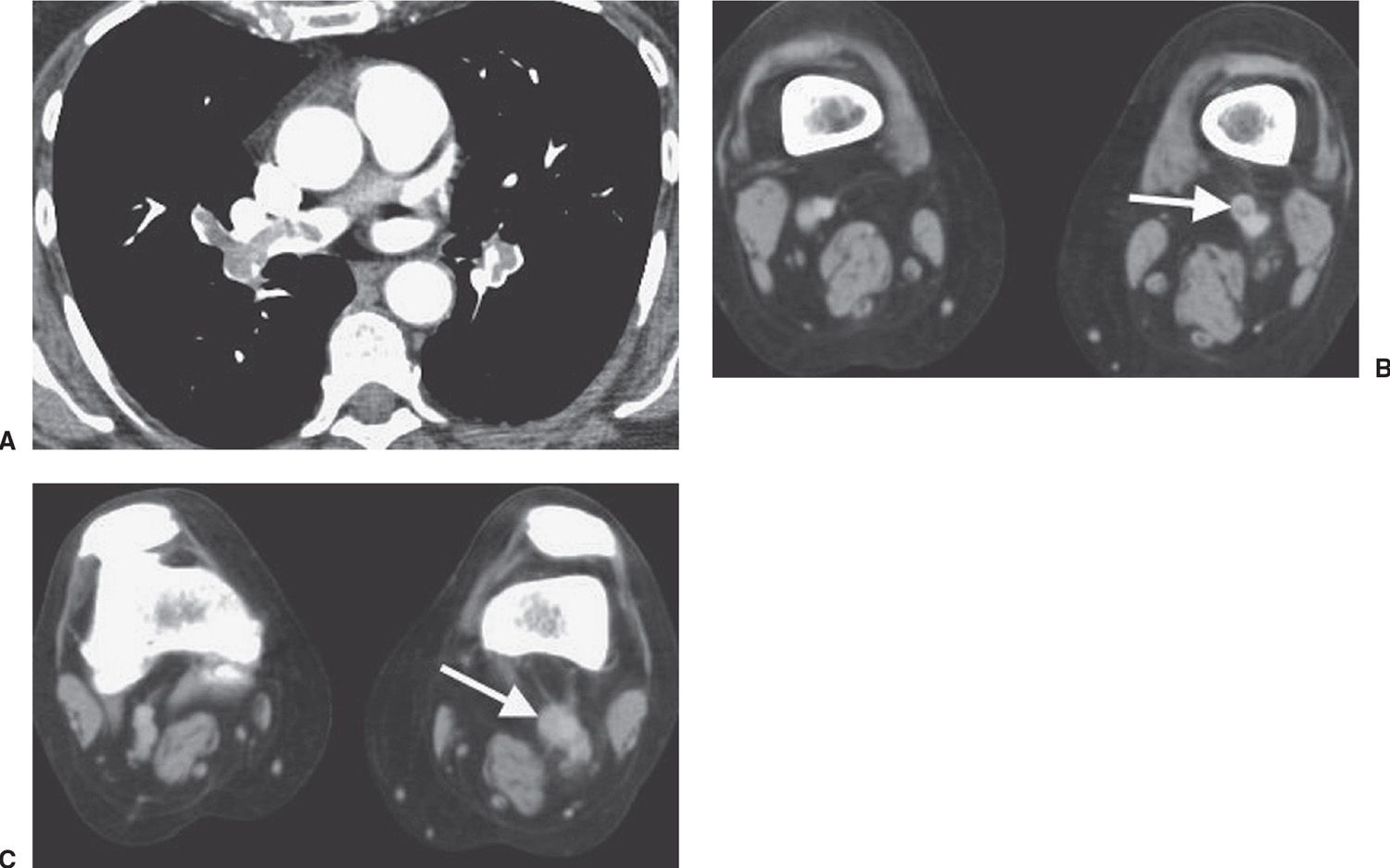
FIG. 17.21 • Deep venous thrombosis and acute PE. A: CTPA of a 66-year-old woman with an endometrial mass and left leg swelling shows bilateral PE. B: CTV performed immediately after the CTPA shows left DVT (arrow). C: CTV at a level inferior to (B) shows expansion of the involved left lower extremity vein and soft tissue stranding of the adjacent fat (arrow).
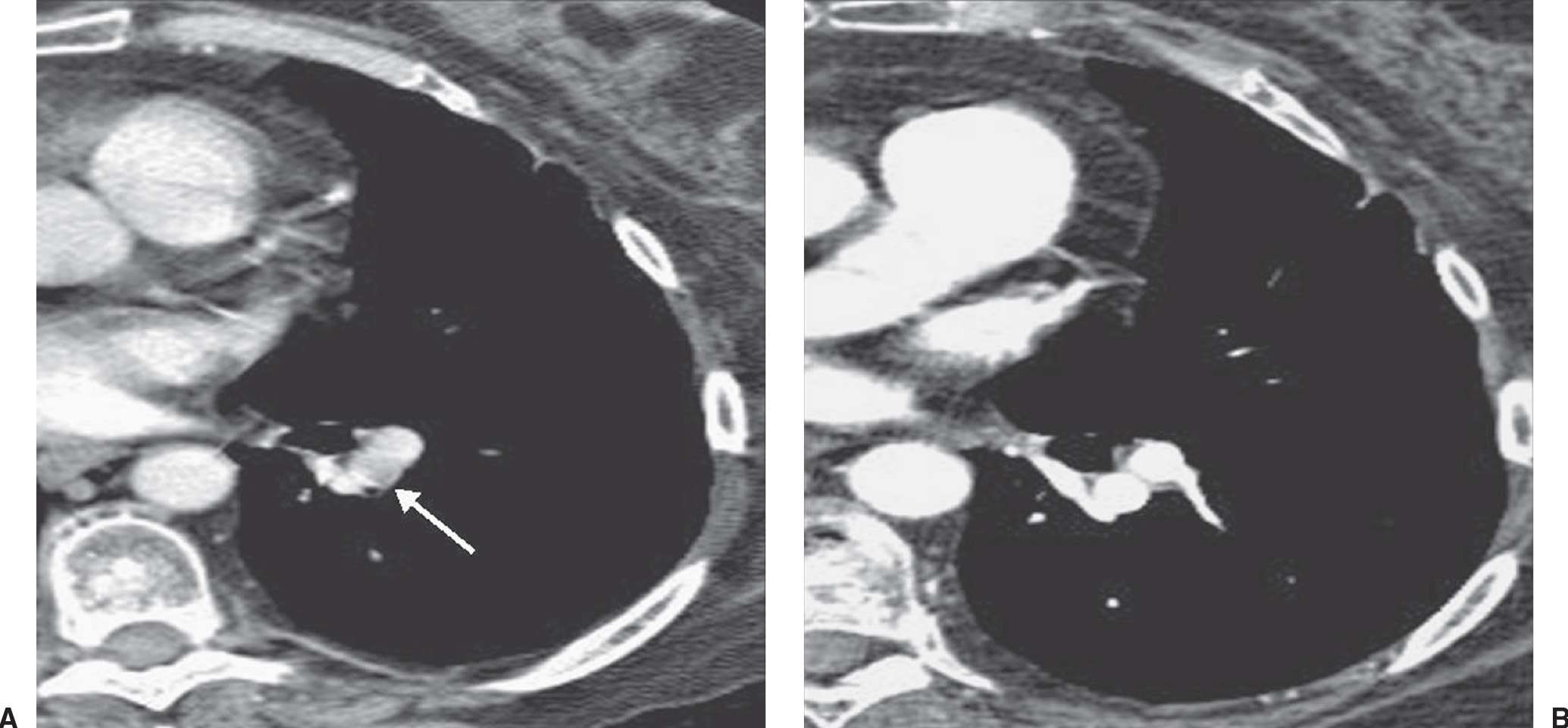
FIG. 17.22 • Partial volume artifact. A: CT with 5-mm collimation shows incomplete enhancement of a left lower lobe segmental pulmonary artery (arrow). B: CTPA on the same day shows homogeneous enhancement of the vessel and no evidence of PE.
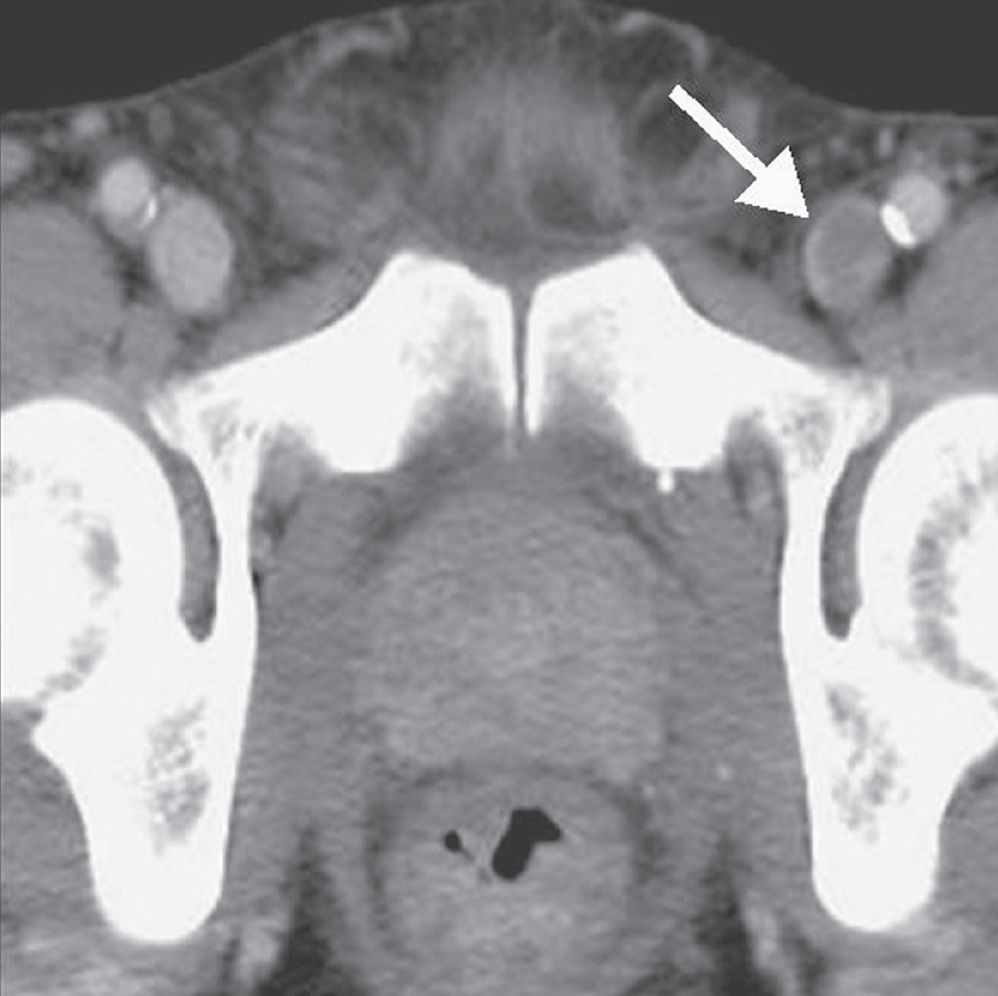
FIG. 17.23 • Deep venous thrombosis. CTV shows intraluminal filling defect within the left femoral vein (arrow).
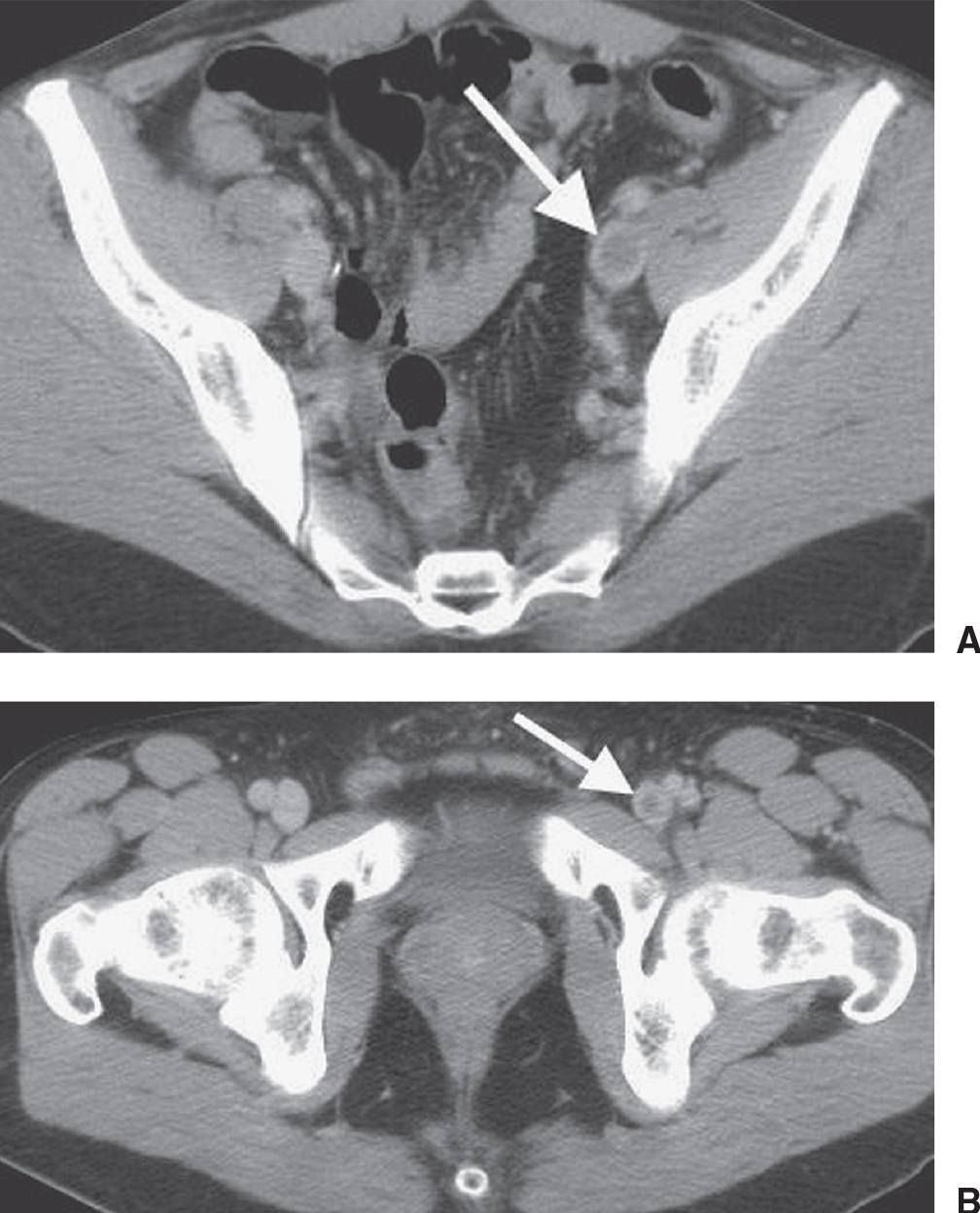
FIG. 17.24 • Deep venous thrombosis. A: CTV of a 42-year-old man with protein C deficiency and recurrent DVT shows a filling defect within a left pelvic vein (arrow). B: CTV at a level inferior to (A) shows thrombus within the left femoral vein (arrow).
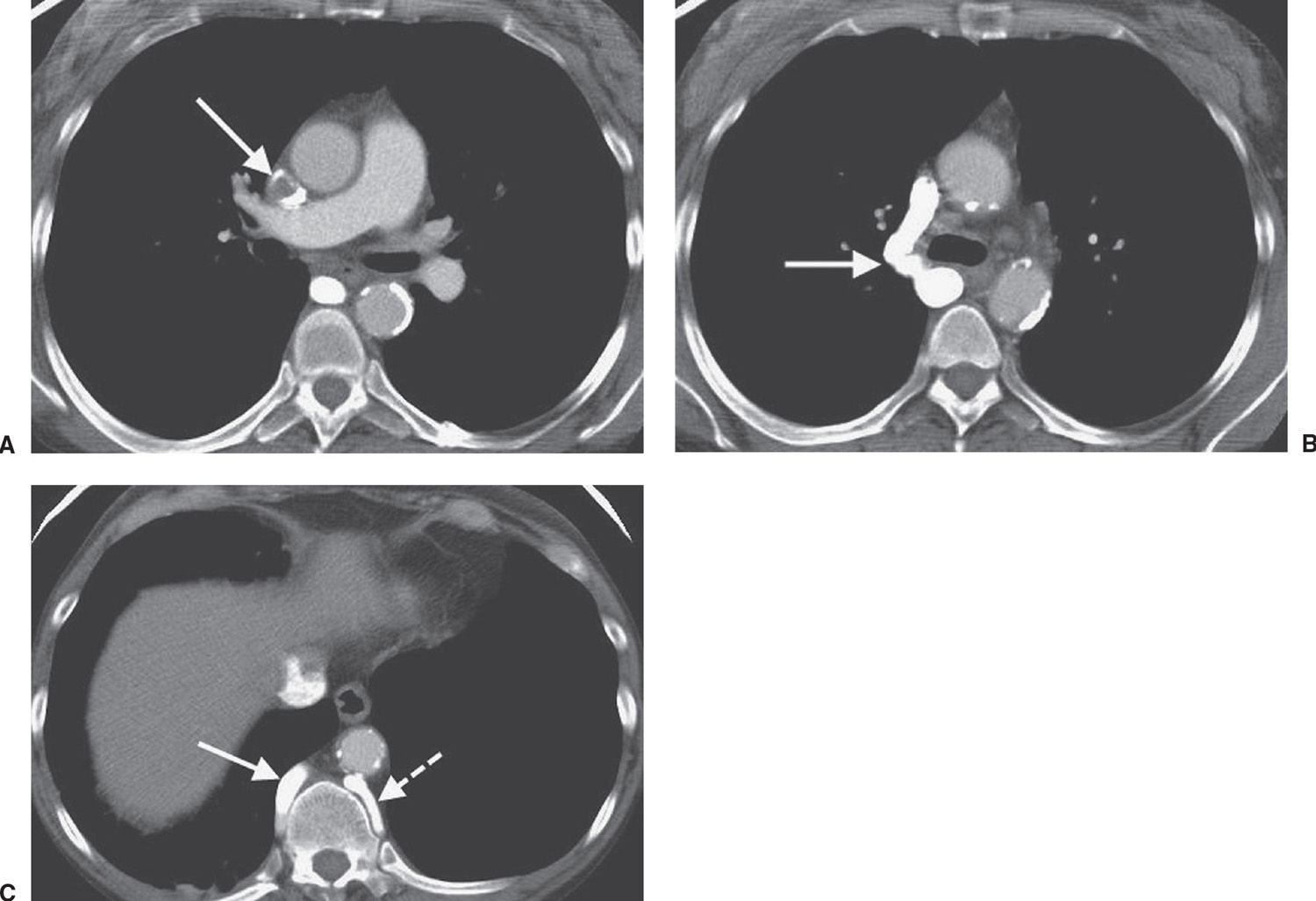
FIG. 17.25 • Superior vena cava thrombus. A: CT of a 47-year-old woman on hemodialysis shows nearly complete occlusion of the superior vena cava with thrombus (arrow). B: CT at a level inferior to (A) shows collateralization of blood flow through an enlarged right azygos vein (arrow). C: CT at a level inferior to (B) shows enlargement of the azygos (solid arrow) and hemiazygos (dashed arrow) veins.
Anticoagulant therapy must be considered for DVT as well as for PE; therefore, ultrasound of the deep venous system should have a primary screening role in patients suspected of PE. If the clinical and/or D-dimer evaluation indicates the need for imaging, the first consideration is whether to perform lower extremity US. When the US is positive, no further testing is needed. If US is negative, PE is not excluded and further testing is needed. Ultrasound imaging has the advantages of being readily available and noninvasive. If ultrasound is negative for DVT, depending on the degree of clinical suspicion, further evaluation is generally obtained with a V/Q scan or CTPA.
The diagnostic feature of PE on a V/Q scan is a perfusion defect in a region of normally ventilated lung—the so-called “mismatched perfusion defect.” Interpretation of V/Q scans is based on a comparison of the V/Q images and the chest radiograph, which gives rise to a report of “normal” or of low, intermediate, or high probability of PE (4). An abnormal V/Q scan indicating a low probability for recent PE is one in which the individual perfusion defects are smaller than 25% of a segment, regardless of the chest radiographic and ventilation scan appearances; are matched on the ventilation scan; or are accompanied by larger chest radiographic abnormalities. A high-probability scan is one in which there are two or more perfusion defects that are not matched by corresponding ventilation defects or chest radiographic abnormalities, including at least one of segmental or larger size. In the appropriate clinical setting, a high-probability V/Q scan indicates a probability of PE exceeding 90%. An intermediate-probability V/Q scan, also described as an indeterminate scan, is an abnormal scan that does not fit into the low- or high-probability categories. It includes those with perfusion defects that, although matched, correspond in size and shape to an area of opacity on the chest radiograph (and, therefore, may represent infarction or pneumonia) or with perfusion defects in areas of severe obstructive lung disease, pulmonary edema, or pleural effusion.
The choice of diagnostic tests depends on the clinical probability of PE, condition of the patient, availability of diagnostic tests, risks of iodinated contrast material, radiation exposure, and cost (Table 17.1). Recommendations can now be made on the basis of the results of the PIOPED II trial and other studies with continued reliance on the physician’s judgment (10). Clinical assessment, using objective criteria, should be made prior to imaging. Clinical decision trees, most notably the Wells criteria, have been developed and validated. The quantitative rapid ELISA, with a sensitivity of 95%, in general has shown the most clinically useful values among the various D-dimer assays (10). No further testing is required if the D-dimer is normal in a patient with a low probability clinical assessment. An abnormal D-dimer in a patient with a low probability clinical assessment indicates the need for further testing with CTPA and/or CTV.
Patients with moderate probability clinical assessment should have a D-dimer rapid ELISA. If negative, no further testing is necessary. If positive, CTPA with or without CTV is recommended. If CTPA is negative, no treatment is necessary.
A D-dimer test is not helpful in patients with high-probability clinical assessment, because it does not exclude PE in more than 15% of such patients (10). CTPA with or without CTV should be performed and if negative, a venous US or MRV is recommended.
Patients with mild-to-moderate iodine allergies may be pretreated with steroids and then imaged with CT. With severe iodine allergy, pulmonary scintigraphy or venous US may be a useful alternative. Venous US is recommended for patients with impaired renal function, and if negative, pulmonary scintigraphy is recommended.
Table 17.1 DIAGNOSTIC ALGORITHM FOR THE EVALUATION OF SUSPECTED PULMONARY EMBOLISM
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree