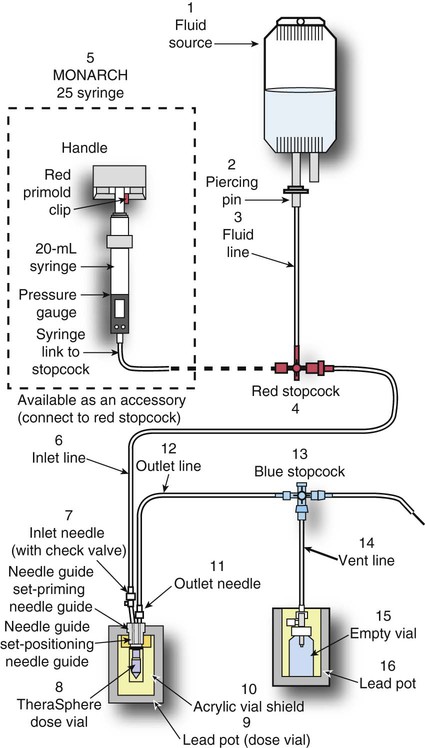
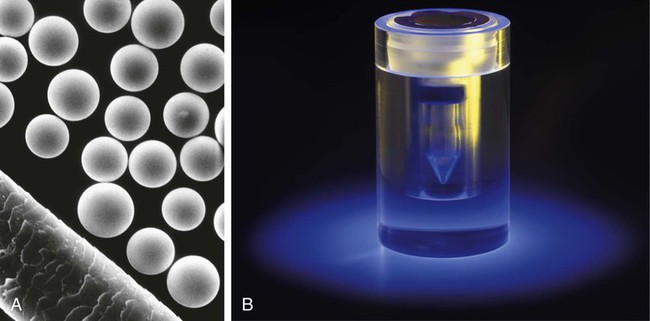
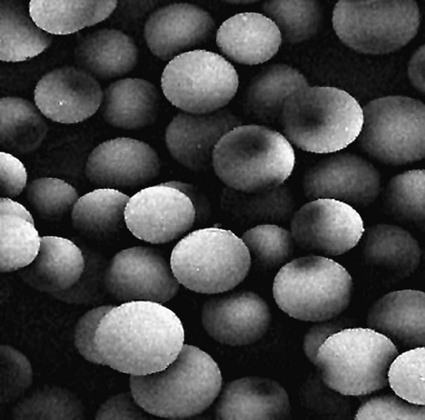
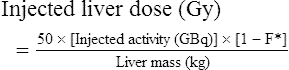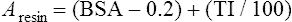Conventional external radiotherapy has been limited and unsatisfactory for hepatocellular carcinoma (HCC), primarily because the liver has a low irradiation tolerance.1 The introduction of three-dimensional conformal radiotherapy (3D-CRT) and other computerized treatment planning techniques have significantly increased the use of radiation therapy in patients with unresectable HCC; however, radiation-induced liver disease (RILD) remains a major complication even when using 3D-CRT.2,3 The treatment of unresectable liver cancer with intraarterial injection of ceramic 90Y microspheres was first introduced in the 1960s.4,5 Early studies focused mainly on treating colorectal liver metastases, but further experience and encouraging results led to the application of 90Y microsphere embolization in patients with HCC.6–8 Currently, there are two types of commercially available radioactive microspheres: • 90Y glass microspheres (TheraSphere [Nordion Inc., Ottawa, Ontario, Canada.]) • 90Y glass resin-based microspheres (SIR-Spheres [Sirtex Medical Inc., Lake Forest, Ill.]) 90Y glass microspheres (TheraSphere) are non-biodegradable insoluble microspheres that contain 90Y in a glass matrix. 90Y cannot leak out from this glass matrix. TheraSphere microspheres have a mean diameter of 25 ± 10 µm and 1 mg contains between 22,000 and 73,000 microspheres (Fig. 65-1). TheraSphere is supplied in 0.6 mL of sterile, pyrogen-free water contained in a 1-mL V-bottom vial secured within a clear acrylic vial shield. TheraSphere is available in six dose sizes: 3 GBq (81 mCi), 5 GBq (135 mCi), 7 GBq (189 mCi), 10 GBq (270 mCi), 15 GBq (405 mCi), and 20 GBq (540 mCi). Each dose of 90Y microspheres is supplied with an administration set that facilitates infusion of the microspheres from the dose vial (see later discussion). The intended dose of radiation to the targeted area ranges between 125 and 150 Gy (12,500-15,000 rads). Following intraarterial injection, TheraSphere microspheres embolize at the arteriole level. Histologic studies have shown increased accumulation of 90Y microspheres along the vascular periphery of the hepatic tumor, and up to 50 or 60 times more than in normal liver parenchyma.9 After lodging in the distal arteriolar circulation, the microspheres emit β radiation that penetrates tissue a maximum effective 10 mm, thereby sparing normal liver parenchyma beyond this limit. Radiation essentially ceases 10 days after embolization, but even before that it poses no threat to others. The second type of 90Y microspheres (SIR-Spheres) is biocompatible, non degradable, and resin-based, with a diameter of 29 to 35 µm (Fig. 65-2). SIR-Spheres have an average activity of 40 Bq per sphere and can be suspended in sterile water and contrast media to the desired total activity. Compared with glass microspheres (specific activity of 2467 Bq per glass microsphere), resin microspheres have much lower specific activity per sphere. SIR-Spheres were granted premarket approval by the FDA in 2002 for treating unresectable metastatic liver tumors from primary colorectal cancer, in conjunction with adjuvant floxuridine-based chemotherapy administered via the hepatic artery. Most clinical trials for treatment of unresectable HCC with SIR-Spheres have been conducted in Australia, Hong Kong, and Europe.10–12 Since the radioactive element is the same as that of glass microspheres, tissue penetration and decay characteristics are identical. Characteristics of the two devices are compared in Table 65-1. The radioactivity of 90Y delivered is dependent on the volume of liver and adjusted for shunting to the gastrointestinal (GI) tract and lungs based on estimates of flow from a technetium-99 (99Tc)-macroaggregated albumin scan. TABLE 65-1 Characteristics of 90Y Microspheres (Glass vs. Resin) FDA, U.S. Food and Drug Administration; HDE, Humanitarian Device Exemption. In an institutional setting, a multidisciplinary panel consisting of interventional radiologists, medical and surgical oncologists, radiation oncologists, hepatologists, pathologists, and transplant surgeons may review patients’ eligibility for treatment with 90Y microspheres. Pretreatment evaluation always includes a routine clinical history, physical examination, complete blood cell count, blood biochemical analysis (including liver and renal function), and an α-fetoprotein assay. Selection criteria are similar to those for transcatheter arterial chemoembolization (TACE) and are listed in Table 65-2. Functional status is assessed by the Eastern Cooperative Oncology Group (ECOG) performance status. Okuda stage and Child-Pugh score should also be obtained before treatment. Pretreatment imaging may include a triple-phase contrast-enhanced spiral computed tomography (CT) scan of the abdomen, chest, and pelvis and/or contrast-enhanced magnetic resonance imaging (MRI) of the abdomen and pelvis to identify extrahepatic disease and calculate tumor and liver volumes. At our institution, the standard imaging workup includes a baseline gadolinium-enhanced MRI scan of the liver and abdomen, with diffusion/perfusion sequences for thoroughly assessing baseline tumor imaging characteristics as well as disease status. TABLE 65-2 Selection Criteria for Radioembolization It bears noting here that patients in whom the hepatopulmonary shunt fraction is greater than 10% of the injected dose with glass microspheres and greater than 20% with resin-based microspheres, or in whom the shunt fraction indicates potential exposure of the lung to an absorbed radiation dose of more than 30 Gy should not be considered for treatment with 90Y microspheres (Fig. 65-3). Calculation of the liver dose (Gy) delivered after injection is provided by the following formula: where Aresin is the activity of the 90Y content of the resin microspheres (in GBq).
Radioembolization for Hepatocellular Carcinoma
Introduction
Yttrium-90 (90Y) Radioactive Microspheres
Clinical Relevance
Glass Microspheres
Resin-Based Microspheres
Mean number of spheres per dose
4 × 106
50 × 106
Specific gravity
3.7 g/cm−3
1.6 g/cm−3
Specific activity
2467 Bq/microsphere
40 Bq/microsphere
FDA approval category
HDE
Premarket approval
Dose variation with tumor volume
No
Yes
Hepatopulmonary shunt upper limit (%)
10
20
Suspension medium
Normal saline
Sterile water
Patient Selection and Preparation

Preoperative Planning
Pretreatment Visceral Angiography and 99mTc-Labeled Macroaggregated Albumin Injection
Radiation Dosimetry