and Ashutosh Dash2
(1)
Nuclear Security and Isotope Division, Oak Ridge National Laboratory, OAK RIDGE, USA
(2)
Isotope Production and Applications Division, Bhabha Atomic Research Centre, Mumbai, India
9.1 Introduction
For the purposes of this discussion, radioisotopes are attached to antibodies for either diagnostic or therapeutic application. The use of antibodies radiolabeled with therapeutic radioisotopes is referred to as radioimmunotherapy (RIT). This technology has evolved from the spectacular advances and growth in the fields of molecular biology and biotechnological. The ability of an antibody to recognize the cognate target antigen with exquisite specificity is the genesis of RIT. In particular, the use of monoclonal antibodies (mAb) radiolabeled with therapeutic radionuclides directed against specific tumor antigens is a unique strategy for site-specific delivery of radiation directly to the tumor, and this represents the cornerstone for the success of RIT. In this context, the invention of hybridoma technology by Kohler and Milstein in 1975 merits special attention (Kohler and Milstein 1975). In 1984, they shared the Nobel Prize for Physiology and Medicine just 9 years after publishing their seminal paper on hybridoma technology. The pioneer research of Kohler and Milstein resulted for the first time in the production of rodent antibodies of single specificity (monoclonal antibodies), and this triggered the use of monoclonal antibodies as delivery vehicles for radionuclides for therapy. RIT combines the synergistic effects of both radiation and immunotherapy with manageable local and systemic side effects. The characteristic and complex interactions between the tumor, host, radionuclide, and the antigen–antibody complex determine the effectiveness of RIT.
9.2 Identification of Cell Surface Markers
The cluster of differentiation (CD) is a protocol used for the identification and investigation of cell surface molecules present on leukocytes. CD molecules can act in numerous ways, often acting as receptors or ligands (the molecule that activates a receptor) important to the cell. The CD nomenclature was proposed and established in the 1st International Workshop and Conference on Human Leukocyte Differentiation Antigens (HLDA) (Zola et al. 2005; Zola and Swart 2005). This system was intended for the classification of the many monoclonal antibodies (mAbs) generated by different laboratories around the world against epitopes on the surface molecules of leukocytes (white blood cells). Since then, its use has expanded to many other cell types, and more than 320 CD unique clusters and subclusters have been identified. The human leukocyte antigen (HLA) is expressed as cell surface molecules/antigens on various immune cells. Through flow cytometry, the CD markers or HLA molecules are identified using mAbs directed against a specific cell surface antigen. Under the current naming system, antigens that are well characterized are assigned an arbitrary number (e.g., CD1, CD2, etc.), whereas molecules that are recognized by just one monoclonal antibody are given the provisional designation “CDw.” Physiologically, CD antigens do not belong in any particular class of molecules, with their functions ranging from cell surface receptions to adhesion molecules. Although initially used for just human leukocytes, the CD molecule naming convention has now been expanded to cover both other species (e.g., mouse) as well as other cell types. Human CD antigens are currently numbered up to CD363. Before discussing RIT in detail, it is helpful to provide a brief introductory discussion on antibodies, antigens, and lymphoma and their roles in RIT.
9.3 Antibodies
Antibodies are immune system-related proteins called immunoglobulins. A typical antibody molecule is made up of four polypeptide chains. The four chains are divided into two identical light chains and two identical heavy chains (Fig. 9.1). An antibody molecule is depicted as Y-shaped molecules with two identical antigen-binding sites at the ends of the arms of the Y. The light and heavy chains contribute to the antigen-binding sites to receptors or specific “antigens.” Each antibody molecule can bind to two identical antigenic determinants. Where the arms meet, the stem of the Y is known as the hinge region. The hinge region allows segmental flexibility of the antibody molecule. The two antigen-binding ends (or amino-terminal ends) of the antibody molecule are called the Fab fragments (for fragment antigen binding), whereas the stem (or carboxyl-terminal end) of the Y is considered to be the Fc fragment (for fragment crystallizable). The Fc region of the antibody molecule is responsible for its biologic properties.
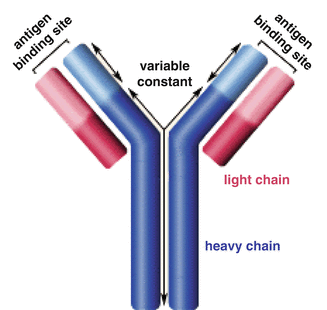

Fig. 9.1
Illustration of antibody structural components
The amino-terminal end of an antibody is called the variable, or V, region and the carboxyl-terminal end is called the constant, or C, region. The C region is about the same size as the V region in the light chain and three to four times larger than the V region in the heavy chain. The V regions of light and heavy chains form the antigen-binding sites. Light and heavy chains consist of repeating, similarly folded homology units or domains. The light chain has one V region (VL) domain and one C region (CL) domain, whereas the heavy chain has one V region (VH) and three or four C region (CH) domains. The most variable parts of the V regions are limited to several small hypervariable regions or complementarity-determining regions (CDRs). The light- and heavy-chain V regions contain three CDRs. The CDRs come together at the amino-terminal end of the antibody molecule to form the antigen-binding site, which determines specificity. The invariant regions of amino acids, between the CDRs, make up about 85 % of the V regions and are designated framework residues. The three functions which explain antibody chemical structure include binding versatility, binding specificity, and biological activity.
9.3.1 B Cells and T Cells
White blood cells (lymphocytes) play an important role in the immune response. While the production of lymphocytes is initiated in bone marrow, after maturation, these circulate through the blood and lymphatic vessels. Lymphocytes fall into the B-cell and the T-cell groups, and each cell is specific for a particular antigen which resides in a receptor for antigen including the B-cell receptor (BCR) for antigen and the T-cell receptor (TCR), respectively. Both BCRs and TCRs are integral membrane proteins displayed on their surface and present in thousands of identical copies exposed at the cell surface, available even before the cell ever encounters an antigen and encoded by genes assembled by the recombination of segments of DNA. However, they differ in structure and the genes that encode them and the type of epitope to which they bind.
Different B cells carry different antibodies, and when a foreign organism invades through the bloodstream, it encounters B cells which express antibodies that can recognize one of the invading antigens. Subsequently, a concerted action follows involving T cells and cells which are called “phagocytes,” where binding of the antigen to the B cell causes further rapid and repeated B-cell division. The resulting collection of daughter cells is called a “clone.” The T cells provide several activities collectively referred to as “cellular immunity,” which is an especially important capability of the body to fight against viruses and to assist B cells for antibody production. The T cells have surface membrane protein receptors which recognize and bind to specific antigens. However, this only occurs when the antigens are attached on cell surfaces. Once the T cells have bound to the antigen, they divide quickly and repeatedly to form an activated clone (Tosato et al. 1980).
9.3.2 Polyclonal and Monoclonal Antibodies
Polyclonal antibody mixtures contain different antibodies developed in the blood of immunized animals from different cell types. Polyclonal antibodies are a combination of immunoglobulin (IgG) molecules secreted against a specific antigen, each identifying a different epitope. As most antigens bear multiple epitopes, they can stimulate the proliferation and differentiation of a variety of B-cell clones. Thus, a heterogeneous pool of serum antibodies can be produced with specificity for particular epitope(s) of the antigen. Polyclonal antibodies recognize multiple epitopes on any one antigen, and the serum obtained will contain a heterogenous complex mixture of antibodies of different affinities. Polyclonals will recognize multiple epitopes on any one antigen and this process has several advantages which include high affinity, since polyclonals can assist in signal amplification from target protein with low expression level, since the target protein will bind more than one antibody molecule on the multiple epitopes. Polyclonal antibody mixtures react with multiple epitopes on the surface of the antigen and will thus be more tolerant of minor changes in the antigen, e.g., polymorphism, heterogeneity of glycosylation, or slight denaturation, than will monoclonal (homogenous) antibodies. Due to recognition of multiple epitopes, polyclonals can provide better results in IP/ChIP and will identify proteins of high homology to the immunogen protein or can be used to screen for the target protein in tissue samples from species other than that of the immunogen. This is because polyclonal antibodies are sometimes used when the nature of the antigen in an untested species is unknown. This capability also makes it important to check immunogen sequence for any cross-reactivity. Often, polyclonal antibodies are the preferred choice for detection of denatured proteins, since multiple epitopes generally provide more robust detection.
9.3.3 Monoclonal Antibodies (mAbs)
In contrast, monoclonal antibodies (mAbs or moAbs) are a mixture of homogenous antibody molecules with affinity towards a specific antigen as they are made by identical immune cells that are several copies of a same parent cell, often generated using a hybridoma by fusing a B cell with a single lineage of cells containing a definite antibody gene (Fig. 9.2).
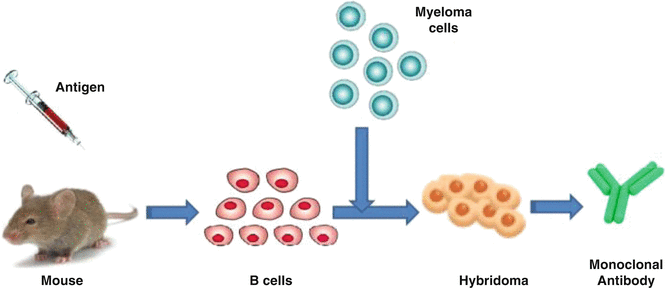

Fig. 9.2
Production of a monoclonal antibody through hybridoma fusion of a B-cell with a single lineage of cells
The availability of mAb through hybridoma technology had revolutionized immunology and has had far reaching implications for a large number of technologies. Monoclonal antibodies have monovalent affinity and homogeneous, in that they bind to the same epitope which makes them effective in therapies. The mAb consists of only one antibody subtype (e.g., IgG1, IgG2, IgG3) that has high specificity, i.e., detects only one epitope on the antigen. As they are more specifically detecting one target epitope, they are less likely to cross-react with other proteins. Compared to polyclonal antibodies, homogeneity of monoclonal antibodies is very high. Specificity of monoclonal antibodies makes them extremely efficient for binding of antigen within a mixture of related molecules. The mAbs are more vulnerable to the loss of epitope through chemical treatment of the antigen than polyclonal antibodies. The basic technique involved in making of monoclonal antibodies relies on fusion of B cells from an immunized mouse with a myeloma (tumor cell line) cell line and let the cells grow in a condition where unfused normal and tumor cells cannot survive. The cells that are fused and able to grow through this procedure are called as hybridomas. Table 9.1 illustrates the key differences between polyclonal and monoclonal antibodies.
Table 9.1
Key differences between polyclonal and monoclonal antibodies
Polyclonal antibodies | Monoclonal antibodies |
|---|---|
Inexpensive to produce | Expensive to produce |
Skills required are low | Training is required for the technology used |
Time scale is short | Time scale is long for hybridomas |
Produces large amounts of nonspecific antibodies | Can produce large amounts of specific antibodies |
Recognizes multiple epitopes on any one antigen | Recognizes only one epitope on an antigen |
Can have batch-to-batch variability | No or low batch-to-batch variability |
Once a hybridoma is made, it is a constant and renewable source |
The success of RIT largely depends on the production of sufficient amounts of monoclonal antibodies which are required for therapy. Table 9.2 provides information for various key antibodies developed for RIT (Fig. 9.3).
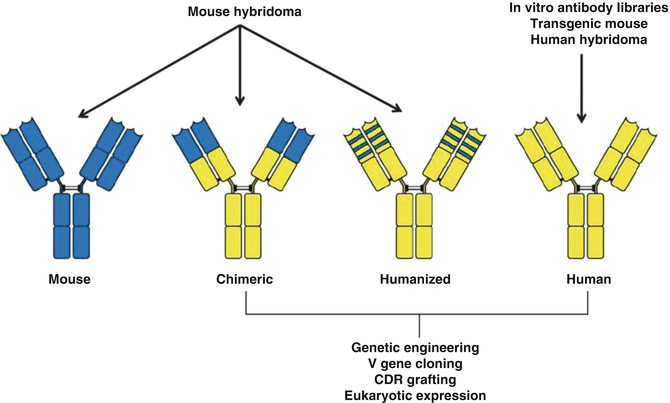
Table 9.2
Examples of antibodies developed for RIT
Antibody designation | Antigen designation | Type of antibody |
|---|---|---|
Lym-1 | HLA-DR10 | Murine IgG2a |
anti-B1 | CD20 | Murine IgG2a |
2B8 | CD20 | Murine IgG1 |
C2B8 | CD20 | Chimeric IgG1 |
hLL2 | CD22 | Humanized IgG1 |
MB-1 | CD37 | Murine IgG1 |
Campath-1H | CD52 | Humanized IgG |

Fig. 9.3
Examples of antibody engineering strategies to obtain “humanized” antibodies
The majority of early clinical studies using radioimmunoconjugates—where therapeutic radioisotopes are attached to antibodies discussed later in—failed to demonstrate a therapeutic impact because monoclonal antibodies of murine origin had been used which are immunogenic in humans and thus prevent repeated administration to patients. Additionally, most radioimmunotherapy approaches for the treatment of solid tumors have proven ineffective because the radiation dose delivered to neoplastic masses was insufficient to induce objective responses and cures. In more recent years, the limitation imposed by the use of monoclonal antibodies of murine origin has been circumvented by the availability and use of more refined chimeric, humanized, and fully human antibodies that can be mass produced (Winter and Harris 1993). The use of techniques to humanize or chimarize monoclonal antibodies to decrease their murine components has been an important advance in this field. These molecules have a long vascular half-life and can interact with human complement or effector cells of the patient immune system. These molecules also behave in a manner similar to naturally occurring immunoglobulin and work along the lines of our normal antibody-based immune response as effective agents in treating patients with cancer (Dillman 2003). Table 9.3 provides information describing a variety of commercially available humanized or chimarized monoclonal antibodies and used in various clinical indications.
Table 9.3
Key examples of commercially available monoclonal antibodies
Monoclonal antibody | Target antigen | Clinical application |
|---|---|---|
OKT3 | CD3 antigens on T lymphocytes | Acute rejection of transplanted kidney, heart, and liver |
Abciximab GP | IIb/IIIa on platelets | Antithrombotic applications |
Rituximab | CD20 receptors on B lymphocytes | Non-Hodgkin’s lymphoma |
Daclizumab | Interleukin-2 receptors on activated T lymphocytes | Acute rejection of transplanted kidneys |
Trastuzumab (Herceptin) | HER-2 growth factor receptors | Advanced breast carcinomas expressing HER-2 receptors |
Infliximab | TNF (tumor necrosis factor) | Rheumatoid arthritis and Crohn’s disease |
Basiliximab | Interleukin-2 receptors on activated T lymphocytes | Acute rejection of transplanted kidney |
Palivizumab | F protein of respiratory syncytial virus (RSV) | RSV infection in children |
Gemtuzumab | CD33 antigen | Relapsed acute myeloid leukemia |
Alemtuzumab | CD52 antigen on B and T lymphocytes | B-cell chronic lymphocytic leukemia |
Cetuximab | EGFR (epidermal growth factor receptor) | Colorectal carcinoma and some other tumors |
9.4 Antigens
An antigen is defined as “any foreign substance that elicits an immune response (e.g., the production of specific antibody molecules) when introduced into the tissues of a susceptible animal and is capable of combining with the specific antibodies formed.” Antigens are generally of high molecular weight and are commonly proteins, carbohydrates, or polysaccharides, lipids, nucleic acids, or even small molecules like neurotransmitters which can act as antigens. These molecules elicit an immune response involving the production of specific antibody molecules when introduced into the tissues of a susceptible animal or human. The antigens are capable of combining with the specific antibodies which are formed. Specific antibodies can interact with only a small region of an antigen, and in the case of a polypeptide, this is generally about 5–12 amino acids. This region can be continuous or it can be distributed in different regions of a primary structure that are brought together because of the secondary or tertiary structure of the antigen. The antigen region which is recognized by an antibody is called an “epitope” and usually consists of one to six monosaccharides or 5–8 amino acid residues on the antigen surface. Because antigen molecules exist in space, the epitope recognized by an antibody may be dependent upon the presence of a specific three-dimensional antigenic conformation. This may be represented by a unique site formed by the interaction of two native protein loops or subunits. In addition, the epitope may correspond to a simple primary sequence region. The range of possible binding sites is enormous, with each potential binding site exhibiting its own structural properties derived from covalent bonds, ionic bonds, and hydrophilic and hydrophobic interactions. For efficient interaction to occur between the antigen and the antibody, the epitope must be readily available for binding. If the target molecule is denatured, e.g., through fixation, reduction, pH changes, or during preparation for gel electrophoresis, the epitope may be altered, and this may affect its ability to interact with an antibody. The epitope may be present in the antigen’s native, cellular environment, or only exposed when denatured. In their natural form, they may be cytoplasmic (soluble), membrane associated, or secreted. The number, location, and size of the epitopes depend on how much of the antigen is presented during the antibody-making process.
There are a large variety of important characteristics of biologic antigens. The most striking feature of antigen–antibody interactions is the high specificity and affinity. It is accepted that almost all the antigens are identified by specific antibodies, but very few have the ability to stimulate the antibodies. Often, it is useful to illicit the formation of antibodies in response to small molecules of interest. However, in contrast to macromolecular molecules which can often illicit the formation of antibodies, small molecules cannot independently provoke an immune response and thus antibody formation. To overcome this, immunologists can attach several copies of small molecules of interest, called “haptens,” to a carrier protein prior to immunization. In this manner, the antibodies which are formed are specific to the hapten/carrier–protein complex. Each antibody binds to a particular part of the antigen called the antigenic determinant (or epitope), which is the specific site on an antigen to which the specific antibody binds. Epitopes are hence also called as antigenic determinants. The random structure on the antigenic molecule that are identified by the antibody as an antigenic binding site thus form the epitope of that antigen. The strength of the antibody–antigen binding is important, and the binding strength between an antigenic determinant in an antigen (epitope) and an antigen-binding site in an antibody (paratope) is referred to as the affinity. Different epitopes can be organized on a single protein molecule in such a manner that their spacing may affect the binding of antibody molecules in various ways. There are a variety of specific terms used in RIT which include the following.
9.4.1 Affinity
This term describes the strength of binding between one antibody-binding site and an antigenic determinant (epitope or hapten). It is the sum of the attractive and repulsive forces, which include van der Waals interactions, hydrogen bonds, salt bridges, and hydrophobic force, although the exact contribution of each of these factors depends on the particular antigen–antibody pair and the combining site of the antibody. The affinity is thus the equilibrium constant that describes the antigen–antibody reaction. The potency of the reaction between a specific antigenic determinant and its single combining site on the antibody determines its affinity. Most antibodies have a high affinity for their antigens.
9.4.2 Avidity
The strength with which a multivalent antibody binds a multivalent antigen is termed avidity, to differentiate it from the affinity of the bond between a single antigenic determinant and an individual combining site. The avidity of an antibody for its antigen is determined by the sum of all of the individual interactions taking place between individual antigen-binding sites of antibodies and determinants on the antigens. The avidity of an antibody for its antigen strongly depends on the affinities of the individual combining sites for the determinants on the antigens. It is controlled by three major factors: antibody epitope affinity, the valence of both the antigen and antibody, and the structural arrangement of the interacting parts. Avidity is more than the sum of the individual affinities.
9.4.3 Specificity
Specificity refers to the ability of an individual antibody-combining site to react with only one antigenic determinant or the ability of a population of antibody molecules to react with only one antigen. In general, there is a high degree of specificity in antigen–antibody reactions. Antibodies can distinguish differences in the primary structure of an antigen, isomeric forms of an antigen, and secondary and tertiary structure of an antigen.
9.4.4 Cross-Reactivity
This term refers to the ability of an individual antibody-combining site to react with more than one antigenic determinant or the ability of a population of antibody molecules to react with more than one antigen. Cross-reactions arise because the cross-reacting antigen shares an epitope in common with the immunizing antigen or because it has an epitope which is structurally similar to one on the immunizing antigen (multispecificity). Normally, antigen–antibody-binding site on antibodies is essentially flat and hence spacious so that they can attach large complexes or structures (Fig. 9.4).
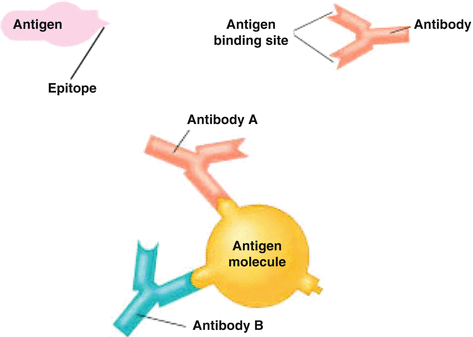

Fig. 9.4
Antibody–antigen binding depends on both specificity and avidity
9.5 Lymphomas
Lymphomas are malignancies of the lymphoid tissue and are broadly classified into Hodgkin’s lymphoma (HD) and non-Hodgkin’s lymphomas (NHL, 85 %). The hallmark of Hodgkin’s lymphoma (HL) is the presence of large, mononucleated Hodgkin and multinucleated Reed/Sternberg cells. These cells represent the tumor cells, but usually comprise less than 1 % of the cellular infiltrate in the lymphoma tissue (Weiss et al. 1999). Due to the rarity of the Hodgkin and Reed/Sternberg (HRS) cells and their unusual phenotype, the origin of these cells from germinal center (GC) B cells in both the lymphocyte predominant (LP) and the classical subtype of HL has only recently been clarified (Küppers 2002). Only in very rare cases do the HRS cells of classical HL represent transformed T cells (Müschen et al. 2000; Seitz et al. 2000).
In contrast, non-Hodgkin’s lymphomas are a heterogeneous group of lymphoreticular malignancies with a wide range of aggressiveness. The majority of NHL are B-cell lymphomas, with the follicular and diffuse large B-cell lymphomas constituting up to 50 % of NHL. NHL can also be classified as indolent (i.e., slow progression; 40 %) or aggressive lymphomas (60 %). B-cell CLL/small lymphocytic lymphoma, marginal zone lymphoma, lymphoplasmacytoid, and follicle center lymphoma constitute the indolent types, whereas diffuse large B-cell, mantle cell, Burkitt’s, and precursor B-cell leukemia constitute the aggressive types. NHL accounts for 4 % of all malignancies and 4 % of all cancer-related deaths (Anand et al. 2008).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree