Vasculitides are a complex group of diseases sharing the defining feature of inflamed vessel walls. Vasculitides can be classified depending on the size of the predominantly affected vessels. Modern cross-sectional imaging methods have become a cornerstone in the diagnosis of vasculitis and may help in narrowing down differential diagnoses. This review presents the most important imaging modalities and typical findings in large and medium size vasculitis, implementing current imaging recommendations.
Key points
- •
Vasculitides can be classified depending on the size of the predominantly affected vessels.
- •
Imaging plays a major role in the diagnosis of vasculitis and may help in narrowing down differential diagnoses. Noninvasive imaging has replaced catheter angiography in patients with suspected large- and medium-vessel vasculitis.
- •
PET and PET/computed tomography enable assessment of disease activity by the increased [ 18 F]-fluorodeoxyglucose uptake of inflamed vessel walls.
- •
The 2018 European League against Rheumatism recommendations recommend color-coded duplex ultrasound and MR imaging as the first imaging modalities in cranial giant cell arteritis and Takayasu’s arteritis.
- •
The extracranial disease extent of giant cell arteritis and Takayasu’s arteritis may be confirmed by PET, computed tomography, MR imaging, or color-coded duplex ultrasound imaging.
Introduction
Vasculitides are a complex group of diseases sharing the defining feature of inflamed vessel walls. Vasculitides can be categorized based on numerous features such as etiology (infectious vs noninfectious), pathogenesis, clinical manifestations, and genetic predispositions. Consequently, the evaluation of patients with suspected vasculitis can be challenging, because the different forms may result in nonspecific symptoms, which may even overlap with those of other diseases.
A key feature for the classification of vasculitides is based on the size and type of the predominantly affected blood vessels ( Fig. 1 ). Radiologic imaging plays a major role in identifying the distribution patterns and extent of the disease. Cross-sectional imaging techniques have gained particular importance in the diagnosis and monitoring of medium- and, especially, large-vessel vasculitides. , In contrast, cross-sectional imaging techniques are limited by their poor visualization of changes in small vessel vasculitis.

This review discusses the most important imaging modalities and typical findings in large- ( Box 1 ) and medium-size vasculitis, implementing current imaging recommendations.
- •
Color-coded duplex ultrasound examination: hypoechogenic, noncompressible “halo” sign, stenosis/occlusion/ectasia
- •
CT, CTA: mural thickening and enhancement, late contrast uptake, stenosis/occlusion/ectasia, surrounding edema/tissue reaction
- •
MR, MRA: mural thickening and enhancement, late contrast uptake, stenosis/occlusion/ectasia, wall/surrounding edema/tissue reaction
- •
PET, PET/CT: mural thickening and tracer uptake, stenosis/occlusion/ectasia > surrounding edema/tissue reaction
- •
CA: stenosis/occlusion/ectasia
Imaging modalities
Color-Coded Duplex Ultrasound Examination
Color-coded duplex ultrasound examination allows for assessment of the vessel lumen and wall, including the surrounding perivascular tissues. Compared with other noninvasive imaging modalities, color-coded duplex ultrasound examination has the highest resolution (0.1 mm), allowing for the detection of inflammatory changes in small arteries.
High-frequency linear transducers with a B-mode frequency of 15 MHz or greater allow for the visualization of small arteries, including the temporal arteries. A B-mode frequency of 7 to 15 MHz should be used for the assessment of extracranial supra-aortic and extremity arteries. A curved array probe with a lower frequency (3.5–5.0 MHz) can be used for assessing large vessels like the aorta and its visceral branches.
Color-coded duplex ultrasound examination does not require ionizing radiation, is widely available, and is less expensive than other cross-sectional imaging modalities. Furthermore, it has been widely used in the assessment of vasculitis, especially of giant cell arteritis (GCA). , In addition, echocardiography is a favorable screening tool for the detection of coronary artery aneurysms in infants with Kawasaki disease (KD). , However, ultrasound examinations are operator dependent and of limited value for visualization of the thoracic aorta as well as inflammatory activity in vasculitis.
Computed Tomography Angiography
Computed tomography angiography (CTA) is widely available and allows for accurate depiction of vessel wall changes owing to its excellent spatial resolution and multiplanar reformations. CTA allows visualization of early changes of vasculitis such as arterial wall thickening, contrast enhancement, and the double ring sign on delayed images. The later changes of vasculitis, such as high attenuation or calcification of the arterial wall, are also readily apparent on CTA.
Vasculitic vessel wall changes are usually smoother and more homogeneous than arteriosclerotic lesions and calcifications are less common. In addition, CTA-derived multiplanar reformations enable accurate diameter measurements in case of late complications of vasculitis such as aneurysms or stenoses. CTA is also of high value in the evaluation of other organs when assessing secondary changes caused by vasculitides, such as parenchymal changes, and to rule out alternative diagnoses.
The European League Against Rheumatism (EULAR) task force recommends CTA be performed with multislice scanners and the following parameters: 120 kV tube voltage, tube current time product (mAs) determined by automatic dose modulation, 0.6-mm collimation, and a reconstruction slice thickness between 0.5 and 1.0 mm. The nonionic iodinated contrast agent (≥350 mg/mL) should be body weight adapted (60–120 mL) and injected with a power injector (≥4 mL/s). Imaging should be performed in the arterial phase defined by a bolus-tracking method (threshold of 100 HU) and in the venous phase (50 s after the arterial phase) using electrocardiogram triggering when imaging the thoracic aorta. Newer dual-energy vascular imaging using variable peak kilovoltage can increase vascular attenuation and image quality. In the opinion of the authors, dual-energy CTA should be performed if available. However, there are not enough studies evaluating the impact of dual-energy CTA in patients with vasculitis. In case of suspected involvement of the coronary arteries in medium vessel vasculitis, such as KD, dedicated coronary CTA provides an accurate assessment of the presence of luminal changes of the coronary arteries. ,
Even though most of the arterial vascular system can be evaluated by CTA, its spatial resolution does not allow for the imaging of small vessels (<0.2 mm). Furthermore, CTA uses ionizing radiation and the application of intravenous contrast media is needed.
PET Combined with Computed Tomography or MR Imaging
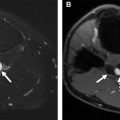
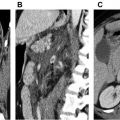
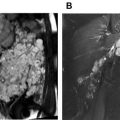
PET is a functional imaging technique that is, widely used in oncology and has also demonstrated a promising role in the study of vasculitis. Combining PET with CT (PET/CT) or CTA gives additional information on wall thickness and luminal changes. When PET/CT is used for imaging of vasculitis, [ 18 F]-fluorodeoxyglucose (FDG) is intravenously applied. FDG-PET/CT detects glucose uptake from high glycolytic activity of inflammatory cells in inflamed arteries, resulting in a pronounced enhancement of vessel walls ( Figs. 2 and 3 ).


Because patients with vasculitis often present with nonspecific symptoms, use of FDG-PET/CT not only helps to identify a suspected vasculitis, but also other pathologies such as infections or oncologic diseases.
However, FDG-PET/CT is expensive, has a lower availability than other imaging methods, and results in radiation exposure. Because atherosclerosis is also an inflammatory disease, inexperienced readers may misinterpret atherosclerotic lesions as large vessel vasculitis. Of note, the vascular uptake of FDG in vasculitis is typically higher than in atherosclerosis. Even though there are no absolute cut-offs values for distinguishing FDG uptake of vasculitis and atherosclerosis a mild vascular FDG uptake (less than or equal to the FDG uptake of the liver), is not indicative of GCA inflammatory involvement. FDG uptake is commonly estimated by visual qualitative methods, but semiquantitative methods such as the vascular/blood ratio and vascular/liver ratio using standardized uptake values are increasingly being used as well.
Poor spatial resolution, poor distinction of brain activity, and a lack of evidence preclude a recommendation for the use of PET/CT in the assessment of inflammation in cranial arteries.
FDG-PET imaging combined with MR imaging (PET/MR imaging) is a promising new technique that combines the functional information derived from the FDG uptake in PET with the high spatial resolution and soft tissue contrast of MR imaging. However, there is currently only limited information on the clinical impact of PET/MR imaging, and its availability is even lower than that of PET/CT.
MR Imaging
MR imaging relies on the intrinsic magnetic properties of body tissues and blood in an external magnetic field, enabling imaging without the need of ionizing radiation or nephrotoxic contrast agents. Detailed depiction of the vasculature can be achieved with MR angiography (MRA), which provides excellent image quality with high spatial resolution.
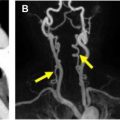
Vasculitis may affect different parts of the vasculature and MRA is an established method for capturing the entire disease extent. MRA is a reliable method for detecting vasculitic changes of large body vessels and superficial cranial arteries. MRA may depict the early inflammatory vasculitic changes with high resolution, including mural thickening and contrast enhancement of the affected vessel (see Fig. 3 ) in addition to luminal stenosis and occlusion.
To evaluate as many affected vessels as possible, the scan range should include the aorta and major branches from the carotid bifurcation to the iliac arteries in coronal acquisition, as well as the axillary and brachial arteries. ,
Because MR imaging itself offers technical flexibility, the EULAR task force recommends standardization of MR imaging protocols to produce sensitive, specific and reliable results.
Imaging with 3.0 T scanners rather than 1.5 T scanners is recommended, when available, to obtain the highest resolution, with a minimum 8-channel head and neck coil and 16-channel body coil. , Settings and technical parameters differ depending on the vasculature to be examined (ie, cranial vessels vs aorta).
Mural inflammation should preferably be assessed using T1-weighted, fat-suppressed, contrast-enhanced sequences and T2-weighted, black blood imaging (eg, navigated 3-dimensional turbo spin echo [TSE], spatial resolution 1.2 × 1.3 × 2 mm 3 , repetition time/echo time of 1000/35 ms). In the presence of mural inflammation, T2-weighted TSE sequences can be helpful for edema detection. However, T2-weighted TSE sequences are less sensitive and more prone to artifacts ,
Time-of-flight angiography can be used for assessment of the intracranial arteries. , Time-resolved contrast-enhanced MRA techniques can be helpful for the evaluation of the large body vessel walls, especially in the case of blood flow alterations owing to stenosis or occlusion. Although time-resolved contrast-enhanced MRA techniques do not allow for the visualization of specific vasculitic changes of the vessel walls, newer sequences like high-resolution T1-weighted 3-dimensional fat-suppressed TSE sequence (volumetric isotropic TSE acquisition) can be combined with contrast-enhanced MRA, which allows simultaneous assessment of lumen and vessel wall. In addition, this 3-dimensional sequence allows for a shorter acquisition time compared with conventional 2-dimensional black blood sequences, as well as multiplanar reconstruction of vessels.
Cardiac MR imaging plays a major role by identifying different patterns of late gadolinium enhancement; it is a common manifestation in several types of vasculitis.
Disadvantages of MRA are the limited availability, long scanning times, dependency on patient’s compliance, and relatively high costs.
Catheter Angiography
Catheter angiography (CA) has been the standard in the diagnosis of large vessel vasculitis for several decades. With its high resolution, CA is also still important for the detection of stenoses or microaneurysms in medium-sized vessel vasculitis. In other diseases it has largely been replaced by noninvasive cross-sectional imaging methods. The major limitation of CA is the lack of information regarding the vessel wall and its surrounding tissue. CA is also invasive with a higher procedural risk compared with noninvasive imaging methods. Furthermore, there is limited availability, it is expensive, operator dependent, needs iodinated contrast agents, and comprises exposure to ionizing radiation. Nowadays, the main indication for CA is a part of vascular interventions such as percutaneous transluminal balloon angioplasty or stenting in patients with vascular stenosis.
Large vessel vasculitis
The Revised International Chapel Hill Consensus Conference defines large vessels as the aorta and its major branches, except for the most distal branches. The 2 major vasculitis entities affecting the large vessels are GCA and Takayasu’s arteritis (TAK). Even though GCA and TAK have some distinctive imaging features, they are both systemic inflammatory diseases, which might result in variable involvement patterns. GCA and TAK also share common histopathologic features, reflecting shared pathways in tissue inflammation.
It has to be noted that an unknown percentage of patients affected by large vessel vasculitis present with atypical involvement of the smaller vessels. This finding is particularly the case in patients with TAK , where medium-size arteries and coronary arteries might also be involved.
Imaging features of TAK and GCA overlap and are not indicative of 1 disease. Stenosis, occlusion, ectasia, mural and surrounding edema, mural thickening, and contrast enhancement in MR imaging or CT, and FDG uptake in PET/CT can occur in either disease.
The main criteria for diagnosis of either TAK or GCA consist of imaging-based identification of different patterns of vessel involvement, in combination with demographic and clinical information.
Giant Cell Arteritis
GCA is the most common form of systemic vasculitis in patients more than 50 years of age and almost never occurs in younger patients. The incidence is highest in Caucasians with European ancestry and is particularly high in northern European populations. The highest incidence is reported in Denmark with an annual incidence of 76.6 per 100,000, whereas lower incidence rates between 2.2 and 10.0 per 100,000 have been reported in other European countries. The incidence in the United States ranges from 19.8 per 100,000 in a population with a European background to 0.36 per 100,000 in those with African American ancestry.
Even though the etiology of GCA remains inconclusive, recent studies indicate a possible association between GCA and the varicella zoster virus.
Clinical characteristics of GCA are not specific and occur with different frequencies. Headache, scalp tenderness, and jaw claudication are considered as common features (30%–80% of patients) whereas less than 20% of patients present with less common features such as ocular symptoms. Some patients (<5%) may even suffer from infrequent features such as tongue claudication or peripheral neuropathy.
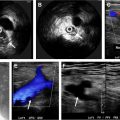
Segmental involvement patterns of the supra-aortic vessels, especially of the cranial arteries (superficial temporal and occipital artery) ( Fig. 4 ), are classic features of GCA. Notably, in up to 40% of patients with extracranial GCA there is no involvement of the temporal arteries. Also, GCA tends to have a predilection for the axillary arteries ( Fig. 5 ) and the EULAR task force recommends including these in the color-coded duplex ultrasound examination assessment in patients with suspected predominantly cranial GCA. ,


A dark, hypoechoic circumferential wall thickening called the halo sign is a key lesion defining vasculitis in patients with suspected GCA. Color-coded duplex ultrasound examination has a pooled sensitivity of 77% and a specificity of 96% for diagnosing GCA in the presence of a halo sign in the temporal arteries. Other features assessed by color-coded duplex ultrasound examination are stenoses and occlusions with consecutive alterations of the flow velocity profile.
MR imaging is able to show distinctive changes of cranial arteries such as mural thickening and contrast enhancement, stenosis, occlusion, ectasia, edema, and sequela such as infarction. MR imaging has a similar diagnostic yield as color-coded duplex ultrasound examination, with a sensitivity of 73% and specificity of 82%. The major limitation of both of these imaging modalities is a decreased sensitivity after treatment initiation with glucocorticoids or immunosuppressive medication.
PET/CT scans has a sensitivity of 80% and a specificity of 89% for detection of GCA and a sensitivity of 88% and specificity of 81% in detecting activity associated with large vessel vasculitis. However, one of the major limitations in the detection of GCA with PET/CT scan is the decrease of diagnostic accuracy after therapy initiation.
The EULAR task force recommends color-coded duplex ultrasound examination and MR imaging as first-line imaging options in patients with suspected GCA ( Table 1 ). Color-coded duplex ultrasound examination is recommended as the first imaging modality in patients where the suspicion is of predominantly cranial GCA, whereas MR imaging may be used as an alternative if color-coded duplex ultrasound examination is not available or inconclusive. The diagnosis of GCA may be made based on imaging findings alone in patients with high clinical suspicion of the disease. In patients with low clinical probability with no imaging findings, the diagnosis can be considered unlikely.